HBV vaccination and PMTCT as elimination tools in the presence of HIV: insights from a clinical cohort and dynamic model
- PMID: 30786896
- PMCID: PMC6383254
- DOI: 10.1186/s12916-019-1269-x
HBV vaccination and PMTCT as elimination tools in the presence of HIV: insights from a clinical cohort and dynamic model
Abstract
Background: Sustainable Development Goals set a challenge for the elimination of hepatitis B virus (HBV) infection as a public health concern by the year 2030. Deployment of a robust prophylactic vaccine and enhanced interventions for prevention of mother to child transmission (PMTCT) are cornerstones of elimination strategy. However, in light of the estimated global burden of 290 million cases, enhanced efforts are required to underpin optimisation of public health strategy. Robust analysis of population epidemiology is particularly crucial for populations in Africa made vulnerable by HIV co-infection, poverty, stigma and poor access to prevention, diagnosis and treatment.
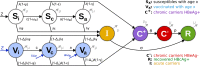
Methods: We here set out to evaluate the current and future role of HBV vaccination and PMTCT as tools for elimination. We first investigated the current impact of paediatric vaccination in a cohort of children with and without HIV infection in Kimberley, South Africa. Second, we used these data to inform a new parsimonious model to simulate the ongoing impact of preventive interventions. By applying these two approaches in parallel, we are able to determine both the current impact of interventions, and the future projected outcome of ongoing preventive strategies over time.
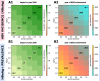
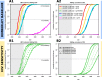
Results: Existing efforts have been successful in reducing paediatric prevalence of HBV infection in this setting to < 1%, demonstrating the success of the existing vaccine campaign. Our model predicts that, if consistently deployed, combination efforts of vaccination and PMTCT can significantly reduce population prevalence (HBsAg) by 2030, such that a major public health impact is possible even without achieving elimination. However, the prevalence of HBV e-antigen (HBeAg)-positive carriers will decline more slowly, representing a persistent population reservoir. We show that HIV co-infection significantly reduces titres of vaccine-mediated antibody, but has a relatively minor role in influencing the projected time to elimination. Our model can also be applied to other settings in order to predict impact and time to elimination based on specific interventions.
Conclusions: Through extensive deployment of preventive strategies for HBV, significant positive public health impact is possible, although time to HBV elimination as a public health concern is likely to be substantially longer than that proposed by current goals.
Keywords: Africa; Antibodies; Elimination; Epidemiology; HIV; Hepatitis B virus; Immunisation; PMTCT; Sustainable Development Goals; Vaccination.
Conflict of interest statement
Ethics approval and consent to participate
Ethics approval was obtained from the Ethics Committee of the Faculty of Health Science, University of the Free State, Bloemfontein, South Africa (HIV Study Ref: ETOVS Nr 08/09 and COSAC Study Ref: ECUFS NR 80/2014), and from the Oxfordshire Research Ethics Committee A, ref 06/Q1604/12. Written consent for enrollment into the study was obtained from the child’s parent/guardian.
Consent for publication
Not applicable.
Competing interests
PCM is an Associate Editor for BMC Infectious Diseases and undertakes consultancy work for Immunocore.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- World Health Organization. Combating hepatitis B and C to reach elimination by 2030: Advocacy Brief 2016.
-
- World Health Organization . Global health sector strategy on viral hepatitis 2016–2021. 2016.
-
- World Health Organization. Introduction of hepatitis B vaccine into childhood immunization services Management guidelines, including information for health workers and parents. Department of Vaccines and Biologicals, https://apps.who.int/iris/handle/10665/66957. 2001.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

