CCDC6 and USP7 expression levels suggest novel treatment options in high-grade urothelial bladder cancer
- PMID: 30786932
- PMCID: PMC6381716
- DOI: 10.1186/s13046-019-1087-1
CCDC6 and USP7 expression levels suggest novel treatment options in high-grade urothelial bladder cancer
Abstract
Background: The muscle invasive form of urothelial bladder cancer (UBC) is a deadly disease. Currently, the therapeutic approach of UBC is mostly based on surgery and standard chemotherapy. Biomarkers to establish appropriate drugs usage are missing. Deficiency of the tumor suppressor CCDC6 determines PARP-inhibitor sensitivity. The CCDC6 levels are modulated by the deubiquitinase USP7. In this work we scored CCDC6 and USP7 expression levels in primary UBC and we evaluated the expression levels of CCDC6 in correlation with the effects of the PARP-inhibitors combined with the USP7 inhibitor, P5091, in vitro. Since PARP-inhibitors could be enhanced by conventional chemotherapy or DNA damage inducers, we tested the new agent RRx-001, able to induce DNA damage, to prove the benefit of combined treatments in bladder cancer cells.
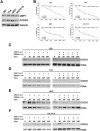
Methods: The J82, T24, 5637 and KU-19-19 bladder cancer cells were exposed to USP7 inhibitor P5091 in presence of cycloheximide to analyse the CCDC6 stability. Upon the CCDC6 degradation induced by P5091, the cells sensitivity to PARP-inhibitor was evaluated by cell viability assays. The ability of the DNA damage inducer RRx-001 to modulate CCDC6 protein levels and H2AX phosphorylation was detected at immunoblot. The combination of USP7 inhibitor plus RRx-001 enhanced the PARP-inhibitor sensitivity, as evaluated by cell viability assays. The results of the scores and correlation of CCDC6 and USP7 expression levels obtained by UBC primary biopsies staining were used to cluster patients by a K-mean cluster analysis.
Results: P5091 determining CCDC6 degradation promoted bladder cancer cells sensitivity to PARP-inhibitor drugs. RRx-001, by inducing DNA damage, enhanced the effects of the combined treatment. The immunohistochemical staining of both CCDC6 and USP7 proteins allowed to cluster the high grade (G3) UBC patients, on the basis of CCDC6 expression levels.
Conclusions: In high grade UBC the identification of two clusters of patients based on CCDC6 and USP7 expession can possibly indicate the use of PARP-inhibitor drugs, in combination with USP7 inhibitor in addition to the DNA damage inducer RRx-001, that also acts as an immunomodulatory agent, offering novel therapeutic strategy for personalized medicine in bladder cancer patients.
Keywords: Biomarkers; DNA damage; DNA repair; Epigenetic; Immunotherapy; P5091; PARP-inhibitor; Precision medicine; RRx-001; Viral mimicry.
Conflict of interest statement
Ethics approval and consent to participate
The study design and procedures involving tissue samples collection and handling were performed according to the Declaration of Helsinki, in agreement with the current Italian law, and to the Institutional Ethical Committee guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

