Induction of labour at 41 weeks versus expectant management until 42 weeks (INDEX): multicentre, randomised non-inferiority trial
- PMID: 30786997
- PMCID: PMC6598648
- DOI: 10.1136/bmj.l344
Induction of labour at 41 weeks versus expectant management until 42 weeks (INDEX): multicentre, randomised non-inferiority trial
Abstract
Objective: To compare induction of labour at 41 weeks with expectant management until 42 weeks in low risk women.
Design: Open label, randomised controlled non-inferiority trial.
Setting: 123 primary care midwifery practices and 45 hospitals (secondary care) in the Netherlands, 2012-16.
Participants: 1801 low risk women with an uncomplicated singleton pregnancy: randomised to induction (n=900) or to expectant management until 42 weeks (n=901).
Interventions: Induction at 41 weeks or expectant management until 42 weeks with induction if necessary.
Primary outcome measures: Primary outcome was a composite of perinatal mortality and neonatal morbidity (Apgar score <7 at five minutes, arterial pH <7.05, meconium aspiration syndrome, plexus brachialis injury, intracranial haemorrhage, and admission to a neonatal intensive care unit (NICU). Secondary outcomes included maternal outcomes and mode of delivery. The null hypothesis that expectant management is inferior to induction was tested with a non-inferiority margin of 2%.
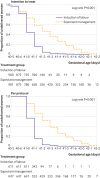
Results: Median gestational age at delivery was 41 weeks+0 days (interquartile range 41 weeks+0 days-41 weeks+1 day) for the induction group and 41 weeks+2 days (41 weeks+0 days-41 weeks+5 days) for the expectant management group. The primary outcome was analysed for both the intention-to-treat population and the per protocol population. In the induction group, 15/900 (1.7%) women had an adverse perinatal outcome versus 28/901 (3.1%) in the expectant management group (absolute risk difference -1.4%, 95% confidence interval -2.9% to 0.0%, P=0.22 for non-inferiority). 11 (1.2%) infants in the induction group and 23 (2.6%) in the expectant management group had an Apgar score <7 at five minutes (relative risk (RR) 0.48, 95% CI 0.23 to 0.98). No infants in the induction group and three (0.3%) in the expectant management group had an Apgar score <4 at five minutes. One fetal death (0.1%) occurred in the induction group and two (0.2%) in the expectant management group. No neonatal deaths occurred. 3 (0.3%) neonates in the induction group versus 8 (0.9%) in the expectant management group were admitted to an NICU (RR 0.38, 95% CI 0.10 to 1.41). No significant difference was found in composite adverse maternal outcomes (induction n=122 (13.6%) v expectant management n=102 (11.3%)) or in caesarean section rate (both groups n=97 (10.8%)).
Conclusions: This study could not show non-inferiority of expectant management compared with induction of labour in women with uncomplicated pregnancies at 41 weeks; instead a significant difference of 1.4% was found for risk of adverse perinatal outcomes in favour of induction, although the chances of a good perinatal outcome were high with both strategies and the incidence of perinatal mortality, Apgar score <4 at five minutes, and NICU admission low.
Trial registration: Netherlands Trial Register NTR3431.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form (available on request from the corresponding author) and declare: BWM is supported by a National Health and Medical Research Council practitioner fellowship (GNT1082548) and reports consultancy for ObsEva, Merck, and Guerbet; no support from any other organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years, no other relationships or activities that could appear to have influenced the submitted work.
Figures
Comment in
-
Optimising the management of late term pregnancies.BMJ. 2019 Feb 20;364:l681. doi: 10.1136/bmj.l681. BMJ. 2019. PMID: 30787009 No abstract available.
References
-
- WHO Collaborating Centers International classification of diseases. Epidemiol Bull 1998;19:7-11. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical