The endoplasmic reticulum (ER) chaperones BiP and Grp94 selectively associate when BiP is in the ADP conformation
- PMID: 30787103
- PMCID: PMC6484115
- DOI: 10.1074/jbc.RA118.007050
The endoplasmic reticulum (ER) chaperones BiP and Grp94 selectively associate when BiP is in the ADP conformation
Abstract
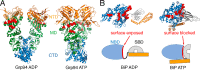
Hsp70 and Hsp90 chaperones are critical for protein quality control in the cytosol, whereas organelle-specific Hsp70/Hsp90 paralogs provide similar protection for mitochondria and the endoplasmic reticulum (ER). Cytosolic Hsp70/Hsp90 can operate sequentially with Hsp90 selectively associating with Hsp70 after Hsp70 is bound to a client protein. This observation has long suggested that Hsp90 could have a preference for interacting with clients at their later stages of folding. However, recent work has shown that cytosolic Hsp70/Hsp90 can directly interact even in the absence of a client, which opens up an alternative possibility that the ordered interactions of Hsp70/Hsp90 with clients could be a consequence of regulated changes in the direct interactions between Hsp70 and Hsp90. However, it is unknown how such regulation could occur mechanistically. Here, we find that the ER Hsp70/Hsp90 (BiP/Grp94) can form a direct complex in the absence of a client. Importantly, the direct interaction between BiP and Grp94 is nucleotide-specific, with BiP and Grp94 having higher affinity under ADP conditions and lower affinity under ATP conditions. We show that this nucleotide-specific association between BiP and Grp94 is largely due to the conformation of BiP. When BiP is in the ATP conformation its substrate-binding domain blocks Grp94; in contrast, Grp94 can readily associate with the ADP conformation of BiP, which represents the client-bound state of BiP. Our observations provide a mechanism for the sequential involvement of BiP and Grp94 in client folding where the conformation of BiP provides the signal for the subsequent recruitment of Grp94.
Keywords: BiP; Grp94; allostery; chaperone; fluorescence anisotropy; fluorescence resonance energy transfer (FRET); heat shock protein 90 (Hsp90); nuclear magnetic resonance (NMR); nucleotide; structural dynamics.
© 2019 Sun et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures






References
-
- Morishima Y., Murphy P. J., Li D. P., Sanchez E. R., and Pratt W. B. (2000) Stepwise assembly of a glucocorticoid receptor·hsp90 heterocomplex resolves two sequential ATP-dependent events involving first hsp70 and then hsp90 in opening of the steroid binding pocket. J. Biol. Chem. 275, 18054–18060 10.1074/jbc.M000434200 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

