Lipopolysaccharide suppresses T cells by generating extracellular ATP that impairs their mitochondrial function via P2Y11 receptors
- PMID: 30787105
- PMCID: PMC6484120
- DOI: 10.1074/jbc.RA118.007188
Lipopolysaccharide suppresses T cells by generating extracellular ATP that impairs their mitochondrial function via P2Y11 receptors
Abstract
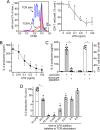
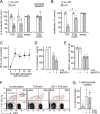
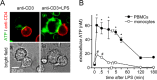
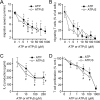
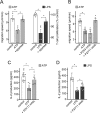
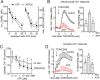
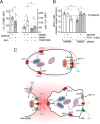
T cell suppression contributes to immune dysfunction in sepsis. However, the underlying mechanisms are not well-defined. Here, we show that exposure of human peripheral blood mononuclear cells to bacterial lipopolysaccharide (LPS) can rapidly and dose-dependently suppress interleukin-2 (IL-2) production and T cell proliferation. We also report that these effects depend on monocytes. LPS did not prevent the interaction of monocytes with T cells, nor did it induce programmed cell death protein 1 (PD-1) signaling that causes T cell suppression. Instead, we found that LPS stimulation of monocytes led to the accumulation of extracellular ATP that impaired mitochondrial function, cell migration, IL-2 production, and T cell proliferation. Mechanistically, LPS-induced ATP accumulation exerted these suppressive effects on T cells by activating the purinergic receptor P2Y11 on the cell surface of T cells. T cell functions could be partially restored by enzymatic removal of extracellular ATP or pharmacological blocking of P2Y11 receptors. Plasma samples obtained from sepsis patients had similar suppressive effects on T cells from healthy subjects. Our findings suggest that LPS and ATP accumulation in the circulation of sepsis patients suppresses T cells by promoting inappropriate P2Y11 receptor stimulation that impairs T cell metabolism and functions. We conclude that inhibition of LPS-induced ATP release, removal of excessive extracellular ATP, or P2Y11 receptor antagonists may be potential therapeutic strategies to prevent T cell suppression and restore host immune function in sepsis.
Keywords: ATP release; T cell; T cell suppression; adaptive immunity; immune dysregulation; lipopolysaccharide (LPS); monocyte; purinergic receptor; purinergic signaling; sepsis.
© 2019 Sueyoshi et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
Similar articles
-
Autocrine regulation of macrophage activation via exocytosis of ATP and activation of P2Y11 receptor.PLoS One. 2013;8(4):e59778. doi: 10.1371/journal.pone.0059778. Epub 2013 Apr 5. PLoS One. 2013. PMID: 23577075 Free PMC article.
-
The purinergic receptor P2Y11 choreographs the polarization, mitochondrial metabolism, and migration of T lymphocytes.Sci Signal. 2020 Sep 29;13(651):eaba3300. doi: 10.1126/scisignal.aba3300. Sci Signal. 2020. PMID: 32994212 Free PMC article.
-
Frontline Science: P2Y11 receptors support T cell activation by directing mitochondrial trafficking to the immune synapse.J Leukoc Biol. 2021 Mar;109(3):497-508. doi: 10.1002/JLB.2HI0520-191R. Epub 2020 Jun 12. J Leukoc Biol. 2021. PMID: 32531829 Free PMC article.
-
Mitochondria Synergize With P2 Receptors to Regulate Human T Cell Function.Front Immunol. 2020 Sep 29;11:549889. doi: 10.3389/fimmu.2020.549889. eCollection 2020. Front Immunol. 2020. PMID: 33133068 Free PMC article. Review.
-
Signal transduction and white cell maturation via extracellular ATP and the P2Y11 receptor.Immunol Cell Biol. 2000 Aug;78(4):369-74. doi: 10.1046/j.1440-1711.2000.00918.x. Immunol Cell Biol. 2000. PMID: 10947861 Review.
Cited by
-
Dietary Supplementation of EGF Ameliorates the Negatively Effects of LPS on Early-Weaning Piglets: From Views of Growth Performance, Nutrient Digestibility, Microelement Absorption and Possible Mechanisms.Animals (Basel). 2021 May 28;11(6):1598. doi: 10.3390/ani11061598. Animals (Basel). 2021. PMID: 34071588 Free PMC article.
-
Signaling Through Purinergic Receptor P2Y2 Enhances Macrophage IL-1β Production.Int J Mol Sci. 2020 Jun 30;21(13):4686. doi: 10.3390/ijms21134686. Int J Mol Sci. 2020. PMID: 32630144 Free PMC article.
-
P2Y11/IL-1 receptor crosstalk controls macrophage inflammation: a novel target for anti-inflammatory strategies?Purinergic Signal. 2023 Sep;19(3):501-511. doi: 10.1007/s11302-023-09932-3. Epub 2023 Apr 5. Purinergic Signal. 2023. PMID: 37016172 Free PMC article. Review.
-
Spatiotemporal transcriptomic profiling and modeling of mouse brain at single-cell resolution reveals cell proximity effects of aging and rejuvenation.bioRxiv [Preprint]. 2024 Jul 19:2024.07.16.603809. doi: 10.1101/2024.07.16.603809. bioRxiv. 2024. PMID: 39071282 Free PMC article. Preprint.
-
P2X receptors in the balance between inflammation and pathogen control in sepsis.Purinergic Signal. 2022 Sep;18(3):241-243. doi: 10.1007/s11302-022-09870-6. Epub 2022 May 25. Purinergic Signal. 2022. PMID: 35612709 Free PMC article. No abstract available.
References
-
- Singer M., Deutschman C. S., Seymour C. W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G. R., Chiche J. D., Coopersmith C. M., Hotchkiss R. S., Levy M. M., Marshall J. C., Martin G. S., Opal S. M., et al. (2016) The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315, 801–810 10.1001/jama.2016.0287 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

