The Antibiotic Neomycin Enhances Coxsackievirus Plaque Formation
- PMID: 30787120
- PMCID: PMC6382971
- DOI: 10.1128/mSphere.00632-18
The Antibiotic Neomycin Enhances Coxsackievirus Plaque Formation
Abstract
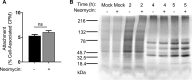
Coxsackievirus typically infects humans via the gastrointestinal tract, which has a large number of microorganisms collectively referred to as the microbiota. To study how the intestinal microbiota influences enteric virus infection, several groups have used an antibiotic regimen in mice to deplete bacteria. These studies have shown that bacteria promote infection with several enteric viruses. However, very little is known about whether antibiotics influence viruses in a microbiota-independent manner. In this study, we sought to determine the effects of antibiotics on coxsackievirus B3 (CVB3) using an in vitro cell culture model in the absence of bacteria. We determined that an aminoglycoside antibiotic, neomycin, enhanced the plaque size of CVB3 strain Nancy. Neomycin treatment did not alter viral attachment, translation, or replication. However, we found that the positive charge of neomycin and other positively charged compounds enhanced viral diffusion by overcoming the negative inhibitory effect of sulfated polysaccharides present in agar overlays. Neomycin and the positively charged compound protamine also enhanced plaque formation of reovirus. Overall, these data provide further evidence that antibiotics can play noncanonical roles in viral infections and that this should be considered when studying enteric virus-microbiota interactions.IMPORTANCE Coxsackieviruses primarily infect the gastrointestinal tract of humans, but they can disseminate systemically and cause severe disease. Using antibiotic treatment regimens to deplete intestinal microbes in mice, several groups have shown the bacteria promote infection with a variety of enteric viruses. However, it is possible that antibiotics have microbiota-independent effects on viruses. Here we show that an aminoglycoside antibiotic, neomycin, can influence quantification of coxsackievirus in cultured cells in the absence of bacteria.
Keywords: antibiotics; coxsackievirus; microbiota; neomycin; reovirus.
Copyright © 2019 Woods Acevedo et al.
Figures





References
-
- Tracy S, Drescher KM, Chapman NM, Kim KS, Carson SD, Pirruccello S, Lane PH, Romero JR, Leser JS. 2002. Toward testing the hypothesis that group B coxsackieviruses (CVB) trigger insulin-dependent diabetes: inoculating nonobese diabetic mice with CVB markedly lowers diabetes incidence. J Virol 76:12097–12111. doi:10.1128/JVI.76.23.12097-12111.2002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
