AMP and GMP Catabolism in Arabidopsis Converge on Xanthosine, Which Is Degraded by a Nucleoside Hydrolase Heterocomplex
- PMID: 30787180
- PMCID: PMC6482636
- DOI: 10.1105/tpc.18.00899
AMP and GMP Catabolism in Arabidopsis Converge on Xanthosine, Which Is Degraded by a Nucleoside Hydrolase Heterocomplex
Abstract
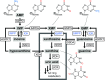
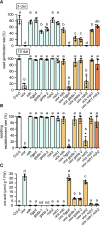
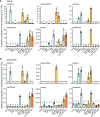
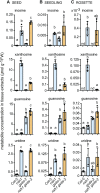
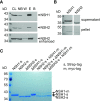
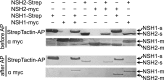
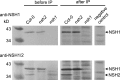
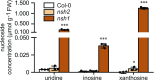
Plants can fully catabolize purine nucleotides. A firmly established central intermediate is the purine base xanthine. In the current widely accepted model of plant purine nucleotide catabolism, xanthine can be generated in various ways involving either inosine and hypoxanthine or guanosine and xanthosine as intermediates. In a comprehensive mutant analysis involving single and multiple mutants of urate oxidase, xanthine dehydrogenase, nucleoside hydrolases, guanosine deaminase, and hypoxanthine guanine phosphoribosyltransferase, we demonstrate that purine nucleotide catabolism in Arabidopsis (Arabidopsis thaliana) mainly generates xanthosine, but not inosine and hypoxanthine, and that xanthosine is derived from guanosine deamination and a second source, likely xanthosine monophosphate dephosphorylation. Nucleoside hydrolase 1 (NSH1) is known to be essential for xanthosine hydrolysis, but the in vivo function of a second cytosolic nucleoside hydrolase, NSH2, is unclear. We demonstrate that NSH1 activates NSH2 in vitro and in vivo, forming a complex with almost two orders of magnitude higher catalytic efficiency for xanthosine hydrolysis than observed for NSH1 alone. Remarkably, an inactive NSH1 point mutant can activate NSH2 in vivo, fully preventing purine nucleoside accumulation in nsh1 background. Our data lead to an altered model of purine nucleotide catabolism that includes an NSH heterocomplex as a central component.
© 2019 American Society of Plant Biologists. All rights reserved.
Figures





References
-
- Alonso J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657. - PubMed
-
- Alseth I., Dalhus B., Bjørås M. (2014). Inosine in DNA and RNA. Curr. Opin. Genet. Dev. 26: 116–123. - PubMed
-
- Ashihara H., Takasawa Y., Suzuki T. (1997). Metabolic fate of guanosine in higher plants. Physiol. Plant. 100: 909–916.
-
- Ashihara H., Stasolla C., Fujimura T., Crozier A. (2018). Purine salvage in plants. Phytochemistry 147: 89–124. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

