Paraffin-enabled graphene transfer
- PMID: 30787292
- PMCID: PMC6382797
- DOI: 10.1038/s41467-019-08813-x
Paraffin-enabled graphene transfer
Abstract
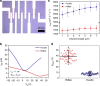
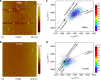
The performance and reliability of large-area graphene grown by chemical vapor deposition are often limited by the presence of wrinkles and the transfer-process-induced polymer residue. Here, we report a transfer approach using paraffin as a support layer, whose thermal properties, low chemical reactivity and non-covalent affinity to graphene enable transfer of wrinkle-reduced and clean large-area graphene. The paraffin-transferred graphene has smooth morphology and high electrical reliability with uniform sheet resistance with ~1% deviation over a centimeter-scale area. Electronic devices fabricated on such smooth graphene exhibit electrical performance approaching that of intrinsic graphene with small Dirac points and high carrier mobility (hole mobility = 14,215 cm2 V-1 s-1; electron mobility = 7438 cm2 V-1 s-1), without the need of further annealing treatment. The paraffin-enabled transfer process could open realms for the development of high-performance ubiquitous electronics based on large-area two-dimensional materials.
Conflict of interest statement
The authors declare no competing interests.
Figures


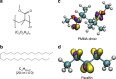

References
-
- Bolotin KI, et al. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008;146:351–355. doi: 10.1016/j.ssc.2008.02.024. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

