Design strategy for serine hydroxymethyltransferase probes based on retro-aldol-type reaction
- PMID: 30787298
- PMCID: PMC6382819
- DOI: 10.1038/s41467-019-08833-7
Design strategy for serine hydroxymethyltransferase probes based on retro-aldol-type reaction
Abstract
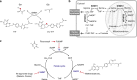
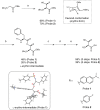
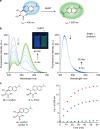
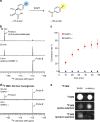
Serine hydroxymethyltransferase (SHMT) is an enzyme that catalyzes the reaction that converts serine to glycine. It plays an important role in one-carbon metabolism. Recently, SHMT has been shown to be associated with various diseases. Therefore, SHMT has attracted attention as a biomarker and drug target. However, the development of molecular probes responsive to SHMT has not yet been realized. This is because SHMT catalyzes an essential yet simple reaction; thus, the substrates that can be accepted into the active site of SHMT are limited. Here, we focus on the SHMT-catalyzed retro-aldol reaction rather than the canonical serine-glycine conversion and succeed in developing fluorescent and 19F NMR molecular probes. Taking advantage of the facile and direct detection of SHMT, the developed fluorescent probe is used in the high-throughput screening for human SHMT inhibitors, and two hit compounds are obtained.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
The crystal structure of human cytosolic serine hydroxymethyltransferase: a target for cancer chemotherapy.Structure. 1998 Sep 15;6(9):1105-16. doi: 10.1016/s0969-2126(98)00112-9. Structure. 1998. PMID: 9753690
-
Serine hydroxymethyltransferase isoforms are differentially inhibited by leucovorin: characterization and comparison of recombinant zebrafish serine hydroxymethyltransferases.Drug Metab Dispos. 2007 Nov;35(11):2127-37. doi: 10.1124/dmd.107.016840. Epub 2007 Jul 30. Drug Metab Dispos. 2007. PMID: 17664250
-
Screening and in vitro testing of antifolate inhibitors of human cytosolic serine hydroxymethyltransferase.ChemMedChem. 2015 Mar;10(3):490-7. doi: 10.1002/cmdc.201500028. Epub 2015 Feb 10. ChemMedChem. 2015. PMID: 25677305 Free PMC article.
-
Structure-function relationship in serine hydroxymethyltransferase.Biochim Biophys Acta. 2003 Apr 11;1647(1-2):24-9. doi: 10.1016/s1570-9639(03)00043-8. Biochim Biophys Acta. 2003. PMID: 12686103 Review.
-
Cloning, expression, activity and folding studies of serine hydroxymethyltransferase: a target enzyme for cancer chemotherapy.J Mol Microbiol Biotechnol. 2003;6(2):67-75. doi: 10.1159/000076737. J Mol Microbiol Biotechnol. 2003. PMID: 15044825 Review.
Cited by
-
Plasma metabolites associated with chronic kidney disease and renal function in adults from the Baltimore Longitudinal Study of Aging.Metabolomics. 2021 Jan 11;17(1):9. doi: 10.1007/s11306-020-01762-3. Metabolomics. 2021. PMID: 33428023 Free PMC article.
-
Therapeutic targeting of the mitochondrial one-carbon pathway: perspectives, pitfalls, and potential.Oncogene. 2021 Apr;40(13):2339-2354. doi: 10.1038/s41388-021-01695-8. Epub 2021 Mar 4. Oncogene. 2021. PMID: 33664451 Review.
-
Universality of critical active site glutamate as an acid-base catalyst in serine hydroxymethyltransferase function.Chem Sci. 2024 Jul 3;15(32):12827-12844. doi: 10.1039/d4sc03187c. eCollection 2024 Aug 14. Chem Sci. 2024. PMID: 39148791 Free PMC article.
-
Metabolic supervision by PPIP5K, an inositol pyrophosphate kinase/phosphatase, controls proliferation of the HCT116 tumor cell line.Proc Natl Acad Sci U S A. 2021 Mar 9;118(10):e2020187118. doi: 10.1073/pnas.2020187118. Proc Natl Acad Sci U S A. 2021. PMID: 33649228 Free PMC article.
-
Synergistic Antibacterial Efficacy of Melittin in Combination with Oxacillin against Methicillin-Resistant Staphylococcus aureus (MRSA).Microorganisms. 2023 Nov 27;11(12):2868. doi: 10.3390/microorganisms11122868. Microorganisms. 2023. PMID: 38138012 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

