Molecular Players in Hematologic Tumor Cell Trafficking
- PMID: 30787933
- PMCID: PMC6372527
- DOI: 10.3389/fimmu.2019.00156
Molecular Players in Hematologic Tumor Cell Trafficking
Abstract
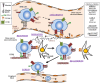
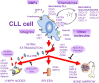
The trafficking of neoplastic cells represents a key process that contributes to progression of hematologic malignancies. Diapedesis of neoplastic cells across endothelium and perivascular cells is facilitated by adhesion molecules and chemokines, which act in concert to tightly regulate directional motility. Intravital microscopy provides spatio-temporal views of neoplastic cell trafficking, and is crucial for testing and developing therapies against hematologic cancers. Multiple myeloma (MM), chronic lymphocytic leukemia (CLL), and acute lymphoblastic leukemia (ALL) are hematologic malignancies characterized by continuous neoplastic cell trafficking during disease progression. A common feature of these neoplasias is the homing and infiltration of blood cancer cells into the bone marrow (BM), which favors growth and survival of the malignant cells. MM cells traffic between different BM niches and egress from BM at late disease stages. Besides the BM, CLL cells commonly home to lymph nodes (LNs) and spleen. Likewise, ALL cells also infiltrate extramedullary organs, such as the central nervous system, spleen, liver, and testicles. The α4β1 integrin and the chemokine receptor CXCR4 are key molecules for MM, ALL, and CLL cell trafficking into and out of the BM. In addition, the chemokine receptor CCR7 controls CLL cell homing to LNs, and CXCR4, CCR7, and CXCR3 contribute to ALL cell migration across endothelia and the blood brain barrier. Some of these receptors are used as diagnostic markers for relapse and survival in ALL patients, and their level of expression allows clinicians to choose the appropriate treatments. In CLL, elevated α4β1 expression is an established adverse prognostic marker, reinforcing its role in the disease expansion. Combining current chemotherapies with inhibitors of malignant cell trafficking could represent a useful therapy against these neoplasias. Moreover, immunotherapy using humanized antibodies, CAR-T cells, or immune check-point inhibitors together with agents targeting the migration of tumor cells could also restrict their survival. In this review, we provide a view of the molecular players that regulate the trafficking of neoplastic cells during development and progression of MM, CLL, and ALL, together with current therapies that target the malignant cells.
Keywords: adhesion molecule; cell trafficking; chemokines (CK); hematological cancer; immunotherapy.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

