Self-assembly as a key player for materials nanoarchitectonics
- PMID: 30787960
- PMCID: PMC6374972
- DOI: 10.1080/14686996.2018.1553108
Self-assembly as a key player for materials nanoarchitectonics
Abstract
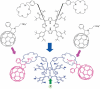
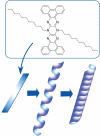
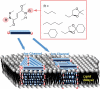
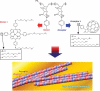
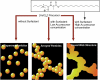
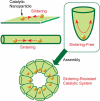
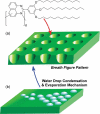
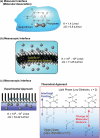
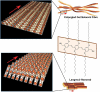
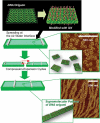
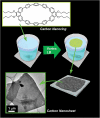
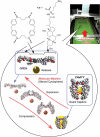
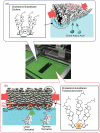
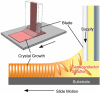
The development of science and technology of advanced materials using nanoscale units can be conducted by a novel concept involving combination of nanotechnology methodology with various research disciplines, especially supramolecular chemistry. The novel concept is called 'nanoarchitectonics' where self-assembly processes are crucial in many cases involving a wide range of component materials. This review of self-assembly processes re-examines recent progress in materials nanoarchitectonics. It is composed of three main sections: (1) the first short section describes typical examples of self-assembly research to outline the matters discussed in this review; (2) the second section summarizes self-assemblies at interfaces from general viewpoints; and (3) the final section is focused on self-assembly processes at interfaces. The examples presented demonstrate the strikingly wide range of possibilities and future potential of self-assembly processes and their important contribution to materials nanoarchitectonics. The research examples described in this review cover variously structured objects including molecular machines, molecular receptors, molecular pliers, molecular rotors, nanoparticles, nanosheets, nanotubes, nanowires, nanoflakes, nanocubes, nanodisks, nanoring, block copolymers, hyperbranched polymers, supramolecular polymers, supramolecular gels, liquid crystals, Langmuir monolayers, Langmuir-Blodgett films, self-assembled monolayers, thin films, layer-by-layer structures, breath figure motif structures, two-dimensional molecular patterns, fullerene crystals, metal-organic frameworks, coordination polymers, coordination capsules, porous carbon spheres, mesoporous materials, polynuclear catalysts, DNA origamis, transmembrane channels, peptide conjugates, and vesicles, as well as functional materials for sensing, surface-enhanced Raman spectroscopy, photovoltaics, charge transport, excitation energy transfer, light-harvesting, photocatalysts, field effect transistors, logic gates, organic semiconductors, thin-film-based devices, drug delivery, cell culture, supramolecular differentiation, molecular recognition, molecular tuning, and hand-operating (hand-operated) nanotechnology.
Keywords: 101 Self-assembly / Self-organized materials; 20 Organic and soft materials (colloids, liquid crystals, gel, polymers); Nanoarchitectonics; interface; nanomaterial; self-assembly.
Figures



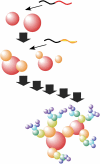









References
-
- Raccuglia P, Elbert KC, Adler PDF, et al. Machine-learning-assisted materials discovery using failed experiments. Nature. 2016;533:73–76. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
