LI-RADS: a conceptual and historical review from its beginning to its recent integration into AASLD clinical practice guidance
- PMID: 30788336
- PMCID: PMC6368120
- DOI: 10.2147/JHC.S186239
LI-RADS: a conceptual and historical review from its beginning to its recent integration into AASLD clinical practice guidance
Abstract
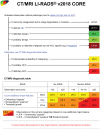
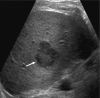
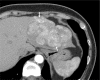
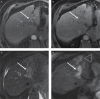
The Liver Imaging Reporting and Data System (LI-RADS®) is a comprehensive system for standardizing the terminology, technique, interpretation, reporting, and data collection of liver observations in individuals at high risk for hepatocellular carcinoma (HCC). LI-RADS is supported and endorsed by the American College of Radiology (ACR). Upon its initial release in 2011, LI-RADS applied only to liver observations identified at CT or MRI. It has since been refined and expanded over multiple updates to now also address ultrasound-based surveillance, contrast-enhanced ultrasound for HCC diagnosis, and CT/MRI for assessing treatment response after locoregional therapy. The LI-RADS 2018 version was integrated into the HCC diagnosis, staging, and management practice guidance of the American Association for the Study of Liver Diseases (AASLD). This article reviews the major LI-RADS updates since its 2011 inception and provides an overview of the currently published LI-RADS algorithms.
Keywords: CEUS; CT; HCC; LI-RADS; MRI; US; cirrhosis; liver imaging; reporting; v2018.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work. The views expressed in this work are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense or the United States Government. Dr Robert M Marks is a military service member. This work was prepared as part of official duties. Title 17 U.S.C. 105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.
Figures




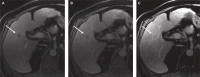



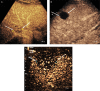


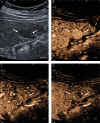
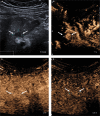
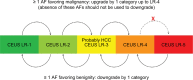

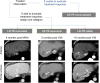
References
-
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750. - PubMed
-
- Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30(1):61–74. - PubMed
-
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. - PubMed
-
- Chernyak V, Santillan CS, Papadatos D, Sirlin CB. LI-RADS® algorithm: CT and MRI. Abdom Radiol (NY) 2018;43(1):111–126. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

