AAV9- TAZ Gene Replacement Ameliorates Cardiac TMT Proteomic Profiles in a Mouse Model of Barth Syndrome
- PMID: 30788385
- PMCID: PMC6369239
- DOI: 10.1016/j.omtm.2019.01.007
AAV9- TAZ Gene Replacement Ameliorates Cardiac TMT Proteomic Profiles in a Mouse Model of Barth Syndrome
Abstract
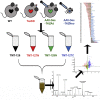
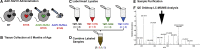
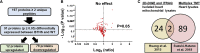
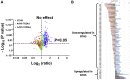
Barth syndrome (BTHS) is a rare mitochondrial disease that causes severe cardiomyopathy and has no disease-modifying therapy. It is caused by recessive mutations in the gene tafazzin (TAZ), which encodes tafazzin-an acyltransferase that remodels the inner mitochondrial membrane lipid cardiolipin. To identify novel mechanistic pathways involved in BTHS and evaluate the effects of gene therapy on proteomic profiles, we performed a multiplex tandem mass tagging (TMT) quantitative proteomics analysis to compare protein expression profiles from heart lysates isolated from BTHS, healthy wild-type (WT), and BTHS treated with adeno-associated virus serotype 9 (AAV9)-TAZ gene replacement as neonates or adults. 197 proteins with ≥2 unique peptides were identified. Of these, 91 proteins were significantly differentially expressed in BTHS compared to WT controls. Cause-effect relationships between tafazzin deficiency and altered protein profiles were confirmed through demonstrated significant improvements in expression levels following administration of AAV9-TAZ. The importance of TMEM65 in Cx43 localization to cardiac intercalated discs was revealed as a novel consequence of tafazzin deficiency that was improved following gene therapy. This study identifies novel mechanistic pathways involved in the pathophysiology of BTHS, demonstrates the ability of gene delivery to improve protein expression profiles, and provides support for clinical translation of AAV9-TAZ gene therapy.
Keywords: AAV9; BTHS; Barth syndrome; Cx43; TMEM65; TMT proteomics; cardiac gene therapy; gap junction localization; mitochondrial disease; tafazzin.
Figures




References
-
- Houtkooper R.H., Turkenburg M., Poll-The B.T., Karall D., Pérez-Cerdá C., Morrone A., Malvagia S., Wanders R.J., Kulik W., Vaz F.M. The enigmatic role of tafazzin in cardiolipin metabolism. Biochim. Biophys. Acta. 2009;1788:2003–2014. - PubMed
-
- Neuwald A.F. Barth syndrome may be due to an acyltransferase deficiency. Curr. Biol. 1997;7:R465–R466. - PubMed
-
- Schlame M., Kelley R.I., Feigenbaum A., Towbin J.A., Heerdt P.M., Schieble T., Wanders R.J., DiMauro S., Blanck T.J. Phospholipid abnormalities in children with Barth syndrome. J. Am. Coll. Cardiol. 2003;42:1994–1999. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

