Laboratory Findings, Compassionate Use of Favipiravir, and Outcome in Patients With Ebola Virus Disease, Guinea, 2015-A Retrospective Observational Study
- PMID: 30788508
- PMCID: PMC6581890
- DOI: 10.1093/infdis/jiz078
Laboratory Findings, Compassionate Use of Favipiravir, and Outcome in Patients With Ebola Virus Disease, Guinea, 2015-A Retrospective Observational Study
Abstract
Background: In 2015, the laboratory at the Ebola treatment center in Coyah, Guinea, confirmed Ebola virus disease (EVD) in 286 patients. The cycle threshold (Ct) of an Ebola virus-specific reverse transcription-polymerase chain reaction assay and 13 blood chemistry parameters were measured on admission and during hospitalization. Favipiravir treatment was offered to patients with EVD on a compassionate-use basis.
Methods: To reduce biases in the raw field data, we carefully selected 163 of 286 patients with EVD for a retrospective study to assess associations between potential risk factors, alterations in blood chemistry findings, favipiravir treatment, and outcome.
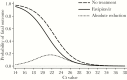
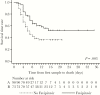
Results: The case-fatality rate in favipiravir-treated patients was lower than in untreated patients (42.5% [31 of 73] vs 57.8% [52 of 90]; P = .053 by univariate analysis). In multivariate regression analysis, a higher Ct and a younger age were associated with survival (P < .001), while favipiravir treatment showed no statistically significant effect (P = .11). However, Kaplan-Meier analysis indicated a longer survival time in the favipiravir-treated group (P = .015). The study also showed characteristic changes in blood chemistry findings in patients who died, compared with survivors.
Conclusions: Consistent with the JIKI trial, this retrospective study revealed a trend toward improved survival in favipiravir- treated patients; however, the effect of treatment was not statistically significant, except for its influence on survival time.
Keywords: Ebola virus disease; Favipiravir; Filovirus; Guinea; epidemic; mobile laboratory.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

References
-
- World Health Organization. Ebola situation report—30 March 2016. http://apps.who.int/ebola/current-situation/ebola-situation-report-30-ma.... Accessed 23 December 2018.
-
- Rollin PE, Bausch DG, Sanchez A. Blood chemistry measurements and D-Dimer levels associated with fatal and nonfatal outcomes in humans infected with Sudan Ebola virus. J Infect Dis 2007; 196(Suppl 2):S364–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

