Demonstration of prion-like properties of mutant huntingtin fibrils in both in vitro and in vivo paradigms
- PMID: 30788585
- PMCID: PMC6531424
- DOI: 10.1007/s00401-019-01973-6
Demonstration of prion-like properties of mutant huntingtin fibrils in both in vitro and in vivo paradigms
Abstract
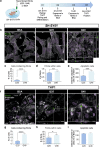
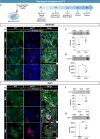
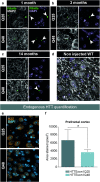
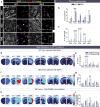
In recent years, evidence has accumulated to suggest that mutant huntingtin protein (mHTT) can spread into healthy tissue in a prion-like fashion. This theory, however, remains controversial. To fully address this concept and to understand the possible consequences of mHTT spreading to Huntington's disease pathology, we investigated the effects of exogenous human fibrillar mHTT (Q48) and huntingtin (HTT) (Q25) N-terminal fragments in three cellular models and three distinct animal paradigms. For in vitro experiments, human neuronal cells [induced pluripotent stem cell-derived GABA neurons (iGABA) and (SH-SY5Y)] as well as human THP1-derived macrophages, were incubated with recombinant mHTT fibrils. Recombinant mHTT and HTT fibrils were taken up by all cell types, inducing cell morphology changes and death. Variations in HTT aggregation were further observed following incubation with fibrils in both THP1 and SH-SY5Y cells. For in vivo experiments, adult wild-type (WT) mice received a unilateral intracerebral cortical injection and R6/2 and WT pups were administered fibrils via bilateral intraventricular injections. In both protocols, the injection of Q48 fibrils resulted in cognitive deficits and increased anxiety-like behavior. Post-mortem analysis of adult WT mice indicated that most fibrils had been degraded/cleared from the brain by 14 months post-surgery. Despite the absence of fibrils at these later time points, a change in the staining pattern of endogenous HTT was detected. A similar change was revealed in post-mortem analysis of the R6/2 mice. These effects were specific to central administration of fibrils, as mice receiving intravenous injections were not characterized by behavioral changes. In fact, peripheral administration resulted in an immune response mounting against the fibrils. Together, the in vitro and in vivo data indicate that exogenously administered mHTT is capable of both causing and exacerbating disease pathology.
Figures


References
-
- Ast A, Buntru A, Schindler F, Hasenkopf R, Schulz A, Brusendorf L, Klockmeier K, Grelle G, McMahon B, Niederlechner H, et al. mHTT seeding activity: a marker of disease progression and neurotoxicity in models of Huntington’s disease. Mol Cell. 2018;71(675–688):e676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

