Complexation with C60 Fullerene Increases Doxorubicin Efficiency against Leukemic Cells In Vitro
- PMID: 30788638
- PMCID: PMC6382919
- DOI: 10.1186/s11671-019-2894-1
Complexation with C60 Fullerene Increases Doxorubicin Efficiency against Leukemic Cells In Vitro
Erratum in
-
Correction to: Complexation with C60 Fullerene Increases Doxorubicin Efficiency against Leukemic Cells In Vitro.Nanoscale Res Lett. 2019 Mar 13;14(1):91. doi: 10.1186/s11671-019-2917-y. Nanoscale Res Lett. 2019. PMID: 30868449 Free PMC article.
Abstract
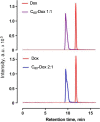
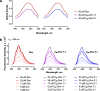
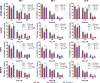
Conventional anticancer chemotherapy is limited because of severe side effects as well as a quickly evolving multidrug resistance of the tumor cells. To address this problem, we have explored a C60 fullerene-based nanosized system as a carrier for anticancer drugs for an optimized drug delivery to leukemic cells.Here, we studied the physicochemical properties and anticancer activity of C60 fullerene noncovalent complexes with the commonly used anticancer drug doxorubicin. C60-Doxorubicin complexes in a ratio 1:1 and 2:1 were characterized with UV/Vis spectrometry, dynamic light scattering, and high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). The obtained analytical data indicated that the 140-nm complexes were stable and could be used for biological applications. In leukemic cell lines (CCRF-CEM, Jurkat, THP1 and Molt-16), the nanocomplexes revealed ≤ 3.5 higher cytotoxic potential in comparison with the free drug in a range of nanomolar concentrations. Also, the intracellular drug's level evidenced C60 fullerene considerable nanocarrier function.The results of this study indicated that C60 fullerene-based delivery nanocomplexes had a potential value for optimization of doxorubicin efficiency against leukemic cells.
Keywords: Accumulation; C60 fullerene; Cytotoxicity; Doxorubicin; Leukemic cells; Noncovalent complex.
Conflict of interest statement
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Kroto HW, Heath JR, O’Brien SC, et al. C60: Buckminsterfullerene. Nature. 1985;318:162–163. doi: 10.1038/318162a0. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

