SMPD1 mutations, activity, and α-synuclein accumulation in Parkinson's disease
- PMID: 30788890
- PMCID: PMC6469643
- DOI: 10.1002/mds.27642
SMPD1 mutations, activity, and α-synuclein accumulation in Parkinson's disease
Abstract
Background: SMPD1 (acid-sphingomyelinase) variants have been associated with Parkinson's disease in recent studies. The objective of this study was to further investigate the role of SMPD1 mutations in PD.
Methods: SMPD1 was sequenced in 3 cohorts (Israel Ashkenazi Jewish cohort, Montreal/Montpellier, and New York), including 1592 PD patients and 975 controls. Additional data were available for 10,709 Ashkenazi Jewish controls. Acid-sphingomyelinase activity was measured by a mass spectrometry-based assay in the New York cohort. α-Synuclein levels were measured in vitro following CRISPR/Cas9-mediated knockout and siRNA knockdown of SMPD1 in HeLa and BE(2)-M17 cells. Lysosomal localization of acid-sphingomyelinase with different mutations was studied, and in silico analysis of their effect on acid-sphingomyelinase structure was performed.
Results: SMPD1 mutations were associated with PD in the Ashkenazi Jewish cohort, as 1.4% of PD patients carried the p.L302P or p.fsP330 mutation, compared with 0.37% in 10,709 Ashkenazi Jewish controls (OR, 3.7; 95%CI, 1.6-8.2; P = 0.0025). In the Montreal/Montpellier cohort, the p.A487V variant was nominally associated with PD (1.5% versus 0.14%; P = 0.0065, not significant after correction for multiple comparisons). Among PD patients, reduced acid-sphingomyelinase activity was associated with a 3.5- to 5.8-year earlier onset of PD in the lowest quartile versus the highest quartile of acid-sphingomyelinase activity (P = 0.01-0.001). We further demonstrated that SMPD1 knockout and knockdown resulted in increased α-synuclein levels in HeLa and BE(2)-M17 dopaminergic cells and that the p.L302P and p.fsP330 mutations impair the traffic of acid-sphingomyelinase to the lysosome.
Conclusions: Our results support an association between SMPD1 variants, acid-sphingomyelinase activity, and PD. Furthermore, they suggest that reduced acid-sphingomyelinase activity may lead to α-synuclein accumulation. © 2019 International Parkinson and Movement Disorder Society.
Keywords: SMPD1; Parkinson's disease; acid sphingomyelinase; genetics; α-synuclein.
© 2019 International Parkinson and Movement Disorder Society.
Conflict of interest statement
Figures
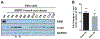

References
-
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 2004;305(5688):1292–1295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K02NS080915/NH/NIH HHS/United States
- Michael J. Fox Foundation for Parkinson's Research/International
- K02 NS080915/NS/NINDS NIH HHS/United States
- U10 NS077267/NS/NINDS NIH HHS/United States
- UL1 TR000040/NH/NIH HHS/United States
- R01 NS101982/NS/NINDS NIH HHS/United States
- Brookdale Foundation/International
- Parkinson's Disease Foundation/International
- R03 NS096494/NS/NINDS NIH HHS/United States
- Canadian Consortium om Neurodegeneration in Aging (CCNA)/International
- Fonds de Recherche du Québec - Santé/International
- UL1 TR000040/TR/NCATS NIH HHS/United States
- R56 NS036630/NS/NINDS NIH HHS/United States
- CIHR/Canada
- R01 NS036630/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

