Treatment Outcomes in Global Systematic Review and Patient Meta-Analysis of Children with Extensively Drug-Resistant Tuberculosis
- PMID: 30789141
- PMCID: PMC6390755
- DOI: 10.3201/eid2503.180852
Treatment Outcomes in Global Systematic Review and Patient Meta-Analysis of Children with Extensively Drug-Resistant Tuberculosis
Abstract
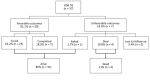
Extensively drug-resistant tuberculosis (XDR TB) has extremely poor treatment outcomes in adults. Limited data are available for children. We report on clinical manifestations, treatment, and outcomes for 37 children (<15 years of age) with bacteriologically confirmed XDR TB in 11 countries. These patients were managed during 1999-2013. For the 37 children, median age was 11 years, 32 (87%) had pulmonary TB, and 29 had a recorded HIV status; 7 (24%) were infected with HIV. Median treatment duration was 7.0 months for the intensive phase and 12.2 months for the continuation phase. Thirty (81%) children had favorable treatment outcomes. Four (11%) died, 1 (3%) failed treatment, and 2 (5%) did not complete treatment. We found a high proportion of favorable treatment outcomes among children, with mortality rates markedly lower than for adults. Regimens and duration of treatment varied considerably. Evaluation of new regimens in children is required.
Keywords: MDR TB; TB; XDR TB; antimicrobial resistance; bacteria; children; extensively drug-resistant tuberculosis; global systematic review; meta-analysis; mortality; multidrug-resistant TB; outcomes; respiratory infections; treatment outcomes; tuberculosis; tuberculosis and other mycobacteria.
Figures



References
-
- World Health Organization. Extensively drug-resistant tuberculosis (XDR-TB): recommendations for prevention and control. Wkly Epidemiol Rec. 2006;81:430–2. - PubMed
-
- World Health Organization. Global Tuberculosis Report 2017. Geneva: The Organization; 2017.
-
- World Health Organization. WHO treatment guidelines for drug-resistant tuberculosis. Geneva: The Organization; 2016. - PubMed

