First-line Nivolumab Plus Ipilimumab vs Sunitinib for Metastatic Renal Cell Carcinoma: A Cost-effectiveness Analysis
- PMID: 30789633
- PMCID: PMC6459127
- DOI: 10.1001/jamaoncol.2018.7086
First-line Nivolumab Plus Ipilimumab vs Sunitinib for Metastatic Renal Cell Carcinoma: A Cost-effectiveness Analysis
Abstract
Importance: Recently, new drugs have been approved for the first-line treatment of metastatic renal cell carcinoma (mRCC). Nivolumab plus ipilimumab significantly increases overall survival for intermediate- and poor-risk patients with mRCC. However, considering the high cost of nivolumab plus ipilimumab, there is a need to assess its value by considering both efficacy and cost.
Objective: To evaluate the cost-effectiveness of nivolumab plus ipilimumab vs sunitinib in the first-line setting for intermediate- and poor-risk patients with mRCC from the US payer perspective.
Design, setting, and participants: A Markov model was developed to compare the lifetime cost and effectiveness of nivolumab plus ipilimumab vs sunitinib in the first-line treatment of mRCC using outcomes data from the CheckMate 214 phase 3 randomized clinical trial, which included 1096 patients with mRCC (median age, 62 years) and compared nivolumab plus ipilimumab vs sunitinib as first-line treatment of mRCC. In the analysis, patients were modeled to receive sunitinib or nivolumab plus ipilimumab for 4 doses followed by nivolumab monotherapy.
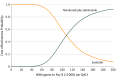
Main outcomes and measures: Life-years, quality-adjusted life-years (QALYs), and lifetime costs were estimated, at a willingness-to-pay threshold of $100 000 to $150 000 per QALY. Univariable, 2-way, and probabilistic sensitivity analyses were performed to evaluate the model uncertainty. Additional subgroup analyses were performed.
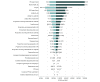
Results: Nivolumab plus ipilimumab provided an additional 0.96 QALYs, at a cost of $108 363 per QALY. Sensitivity analyses found the results to be most sensitive to overall survival hazard ratio (0.63; 95% CI, 0.44-0.89) and mean patient weight (70 kg, range, 40-200 kg). Other variables, such as the cost of nivolumab plus ipilimumab (mean, $32 213.44; range, $25 770.75-$38 656.13), utility values for nivolumab plus ipilimumab (mean, 0.82; range, 0.65-0.98), and proportion receiving nivolumab in sunitinib arm (mean, 0.27; range, 0.22-0.32), had a moderate or minor influence on model results. Subgroup analyses demonstrated that nivolumab plus ipilimumab was most cost-effective for patients with programmed cell death 1 ligand 1 expression of at least 1% ($86 390 per QALY).
Conclusions and relevance: In this model, nivolumab plus ipilimumab was estimated to be cost-effective compared with sunitinib for intermediate- and poor-risk patients with mRCC at a willingness-to-pay threshold from $100 000 to $150 000 per QALY.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

