Iron-Catalyzed Hydroamination and Hydroetherification of Unactivated Alkenes
- PMID: 30789740
- PMCID: PMC6583794
- DOI: 10.1021/acs.orglett.9b00427
Iron-Catalyzed Hydroamination and Hydroetherification of Unactivated Alkenes
Abstract
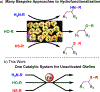
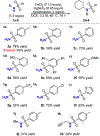
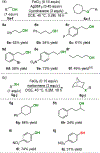
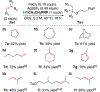
The hydrofunctionalization of alkenes, explored for over 100 years, offers the potential for a direct, atom-economical approach to value-added products. While thermodynamically favored, the kinetic barrier to such processes necessitates the use of catalysts to control selectivity and reactivity. Modern variants typically rely on noble metals that require different ligands for each class of hydrofunctionalization, thereby limiting generality. This Letter describes a general iron-based system that catalyzes the hydroamination and hydroetherification of simple unactivated olefins.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Johns AM; Sakai N; Ridder A; Hartwig JF J. Am. Chem. Soc 2006, 128, 9306–9307. - PubMed
-
- Müller TE; Hultzsch KC; Yus M; Foubelo F; Tada M Chem. Rev 2008, 108, 3795–3892. - PubMed
-
- Hintermann L Recent Developments in Metal-Catalyzed Additions of Oxygen Nucleophiles to Alkenes and Alkynes In C-X Bond Formation; Vigalok A, Ed.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2010; pp 123–155.
-
- Kondo T; Mitsudo T.-a. Chem. Rev 2000, 100, 3205–3220. - PubMed
-
- Huang L; Arndt M; Gooßen K; Heydt H; Gooßen L J. Chem. Rev 2015, 115, 2596–2697. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

