Wnt Signaling Mediates the Aging-Induced Differentiation Impairment of Intestinal Stem Cells
- PMID: 30790135
- PMCID: PMC6534527
- DOI: 10.1007/s12015-019-09880-9
Wnt Signaling Mediates the Aging-Induced Differentiation Impairment of Intestinal Stem Cells
Abstract
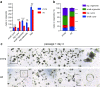
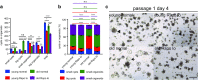
Stem cell aging underlies aging-associated disorders, such as steeply increased incidences of tumors and impaired regeneration capacity upon stress. However, whether and how the intestinal stem cells age remains largely unknown. Here we show that intestinal stem cells derived from 24-month-old mice hardly form typical organoids with crypt-villus structures, but rather mainly form big, rounded cysts devoid of differentiated cell types, which mimics the culturing of heterozygous APC-deficient cells from the APCmin mouse line. Further analysis showed that cultured crypts derived from aged mice exhibited reduced expression levels of differentiation genes and higher expression of Wnt target genes. Lowering the concentration of R-spondin-1 in the culture system significantly reduced formation of rounded cysts, accompanied by an increased formation of organoids from crypts derived from old mice. We are the first to uncover that intestinal stem cells derived from old mice harbor significant deficiency in differentiation that can be partially rescued through a reduction in R-spondin-1 exposure. This could be highly relevant to intestinal tumor development and the reduced regeneration potential observed in the aged population. Our study provides the first experimental evidence that an over-responsiveness to Wnt/beta-catenin signaling of aged intestinal stem cells mediates the aging-induced deficiency in differentiation, and could serve as a potential target to ameliorate aging-associated intestinal pathologies.
Keywords: Aging; Differentiation; Intestinal stem cells; R-spondin-1; Wnt signaling.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

