Pathogenesis of age-related HIV neurodegeneration
- PMID: 30790184
- PMCID: PMC6703984
- DOI: 10.1007/s13365-019-00728-z
Pathogenesis of age-related HIV neurodegeneration
Abstract
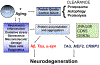
People over the age of 50 are the fastest growing segment of the HIV-infected population in the USA. Although antiretroviral therapy has remarkable success controlling the systemic HIV infection, HIV-associated neurocognitive disorder (HAND) prevalence has increased or remained the same among this group, and cognitive deficits appear more severe in aged patients with HIV. The mechanisms of HAND in the aged population are not completely understood; a leading hypothesis is that aged individuals with HIV might be at higher risk of developing Alzheimer's disease (AD) or one of the AD-related dementias (ADRD). There are a number of mechanisms through which chronic HIV disease alone or in combination with antiretroviral therapy and other comorbidities (e.g., drug use, hepatitis C virus (HCV)) might be contributing to HAND in individuals over the age of 50 years, including (1) overlapping pathogenic mechanisms between HIV and aging (e.g., decreased proteostasis, DNA damage, chronic inflammation, epigenetics, vascular), which could lead to accelerated cellular aging and neurodegeneration and/or (2) by promoting pathways involved in AD/ADRD neuropathogenesis (e.g., triggering amyloid β, Tau, or α-synuclein accumulation). In this manuscript, we will review some of the potential common mechanisms involved and evidence in favor and against a role of AD/ADRD in HAND.
Keywords: Aging; Alzheimer’s disease; HIV-associated cognitive impairment; Neurodegeneration.
Figures


References
-
- (2005). HIV/AIDS surveillance report. 1–63.
-
- (2007). HIV/AIDS surveillance report. 1–54.
-
- Alakkas A, Ellis RJ, Watson CW, Umlauf A, Heaton RK, Letendre S, Collier A, Marra C, Clifford DB, Gelman B, Sacktor N, Morgello S, Simpson D, McCutchan JA, Kallianpur A, Gianella S, Marcotte T, Grant I, Fennema-Notestine C, Group C (2018). White matter damage, neuroinflammation, and neuronal integrity in HAND. Journal of neurovirology: 1–10. - PMC - PubMed

