Electroporation-Mediated Immunization of a Candidate DNA Vaccine Expressing Dengue Virus Serotype 4 prM-E Antigen Confers Long-Term Protection in Mice
- PMID: 30790202
- PMCID: PMC6420567
- DOI: 10.1007/s12250-019-00090-8
Electroporation-Mediated Immunization of a Candidate DNA Vaccine Expressing Dengue Virus Serotype 4 prM-E Antigen Confers Long-Term Protection in Mice
Abstract
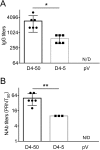
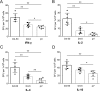
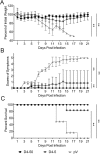
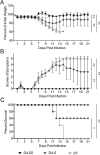
Dengue fever, caused by dengue viruses (DENVs), is a widespread mosquito-borne zoonotic disease; however, there is no available anti-dengue vaccine for worldwide use. In the current study, a DNA vaccine candidate (pV-D4ME) expressing prM-E protein of DENV serotype 4 (DENV-4) was constructed, and its immunogenicity and protection were evaluated in immunocompetent BALB/c mice. The pV-D4ME candidate vaccine induced effective humoral and cellular immunity of mice against DENV-4 in vivo when administered both at 50 μg and 5 μg through electroporation. Two weeks after receiving three immunizations, both doses of pV-D4ME DNA were shown to confer effective protection against lethal DENV-4 challenge. Notably, at 6 months after the three immunizations, 50 μg, but not 5 μg, of pV-D4ME could provide stable protection (100% survival rate) against DENV-4 lethal challenge without any obvious clinical signs. These results suggest that immunization with 50 μg pV-D4ME through electroporation could confer effective and long-term protection against DENV-4, offering a promising approach for development of a novel DNA vaccine against DENVs.
Keywords: DNA vaccine; Dengue virus (DENV); Electroporation; Long-term protection; Mouse.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflict of interest.
Animal and Human Rights Statement
The whole study was approved by the Institutional Animal Care and Use Committee of Capital Medical University, China (Approval Number: AEEI-2015-066). All institutional and national guidelines for the care and use of laboratory animals were followed.
Figures


References
-
- Babiuk S, Baca-Estrada ME, Foldvari M, Middleton DM, Rabussay D, Widera G, Babiuk LA. Increased gene expression and inflammatory cell infiltration caused by electroporation are both important for improving the efficacy of DNA vaccines. J Biotechnol. 2004;110:1–10. doi: 10.1016/j.jbiotec.2004.01.015. - DOI - PubMed
-
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. - DOI - PMC - PubMed
-
- Chen H, Zheng X, Wang R, Gao N, Sheng Z, Fan D, Feng K, Liao X, An J. Immunization with electroporation enhances the protective effect of a DNA vaccine candidate expressing prME antigen against dengue virus serotype 2 infection. Clin Immunol. 2016;171:41–49. doi: 10.1016/j.clim.2016.08.021. - DOI - PubMed
-
- Clements DE, Coller BA, Lieberman MM, Ogata S, Wang G, Harada KE, Putnak JR, Ivy JM, McDonell M, Bignami GS, Peters ID, Leung J, Weeks-Levy C, Nakano ET, Humphreys T. Development of a recombinant tetravalent dengue virus vaccine: immunogenicity and efficacy studies in mice and monkeys. Vaccine. 2010;28:2705–2715. doi: 10.1016/j.vaccine.2010.01.022. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

