Retrospective efficacy analysis of immune checkpoint inhibitors in patients with EGFR-mutated non-small cell lung cancer
- PMID: 30790471
- PMCID: PMC6488155
- DOI: 10.1002/cam4.2037
Retrospective efficacy analysis of immune checkpoint inhibitors in patients with EGFR-mutated non-small cell lung cancer
Abstract
Background: Treatment with epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) leads to initial response in most patients with EGFR-mutated non-small cell lung cancer (NSCLC). In contrast, little is known of the subpopulation of patients with NSCLC with EGFR mutations who exhibit clinical outcomes that require treatment with immune checkpoint inhibitors (ICIs). Therefore, to identify eligible cases to treat with ICIs, we retrospectively analyzed the correlation between clinical features and the efficacy of ICIs in patients with EGFR mutations.
Patients and methods: We retrospectively analyzed patients with advanced NSCLC harboring EGFR mutations who were treated with ICIs after developing resistance to EGFR-TKIs between February 2016 and April 2018 at 6 institutions in Japan. The association between clinical outcomes and the efficacy of ICIs was investigated.
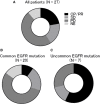
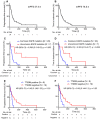
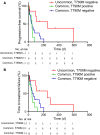
Results: We enrolled 27 patients who harbored EGFR-activating mutations. The objective response and disease control rates were higher in patients with uncommon EGFR mutations than in those with common EGFR mutations (71% vs 35.7% and 57% vs 7%, P = 0.14 and P < 0.01, respectively). Patients with uncommon EGFR mutations or without T790M mutations exhibited a significantly longer median progression-free survival than those with common EGFR mutations or with T790M mutations (P = 0.003 and P = 0.03, respectively).
Conclusion: Patients with uncommon EGFR mutations and without T790M mutations are associated with the best outcomes for treatment with immunotherapy among those with EGFR-mutated NSCLC, based on retrospective analysis. Further research is needed to validate the clinical biomarkers involved in ICI responders with EGFR mutations.
Keywords: EGFR mutation; biomarker; immunology; non-small cell lung cancer.
© 2019 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
All authors have no conflict of interest to declare.
Figures
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics for Hispanics/Latinos, 2012. CA Cancer J Clin. 2012;62(5):283‐298. - PubMed
-
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380‐2388. - PubMed
-
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non‐small‐cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121‐128. - PubMed
-
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802): a multicentre, open‐label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735‐742. - PubMed
-
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327‐3334. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

