Poly(ethylene glycol)-block-poly(d,l-lactic acid) micelles containing oligo(lactic acid)8-paclitaxel prodrug: In Vivo conversion and antitumor efficacy
- PMID: 30790593
- PMCID: PMC6466635
- DOI: 10.1016/j.jconrel.2019.02.017
Poly(ethylene glycol)-block-poly(d,l-lactic acid) micelles containing oligo(lactic acid)8-paclitaxel prodrug: In Vivo conversion and antitumor efficacy
Abstract
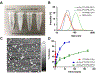
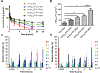
Poly(ethylene glycol)-block-poly(d,l-lactic acid) (PEG-b-PLA) micelles affect drug solubilization, and a paclitaxel (PTX) loaded-PEG-b-PLA micelle (PTX-PM) is approved for cancer treatment due to injection safety and dose escalation (Genexol-PM®) compared to Taxol®. However, PTX-PM is unstable in blood, has rapid clearance, and causes dose-limiting toxicity. We have synthesized a prodrug for PTX (7-OH), using oligo(lactic acid) as a novel pro-moiety (o(LA)8-PTX) specifically for PEG-b-PLA micelles, gaining higher loading and slower release of o(LA)8-PTX over PTX. Notably, o(LA)8-PTX prodrug converts into PTX by a backbiting reaction in vitro, without requiring esterases. We hypothesize that o(LA)8-PTX-loaded PEG-b-PLA micelles (o(LA)8-PTX-PM) has a lower Cmax and higher plasma AUC than PTX-PM for improved therapeutic effectiveness. In Sprague-Dawley rats at 10 mg/kg, compared to o(LA)8-PTX-PM (10% w/w loading) and PTX-PM (10%), o(LA)8-PTX-PM (50% w/w loading) produces a 2- and 3-fold higher plasma AUC0-24 of PTX, lactic acid-PTX, and o(LA)2-PTX (o(LA)0-2-PTX), respectively. For o(LA)8-PTX-PM at 10 and 50% w/w loading, PTX and lactic acid-PTX are major bioactive metabolites, respectively. Fast prodrug conversion of o(LA)8-PTX in vivo versus in vitro (by backbiting) suggests that o(LA)8 is a good substrate for esterases. At 60 mg/kg (qwx3), o(LA)8-PTX-PM (50%) has higher antitumor activity than o(LA)8-PTX-PM (10%) and PTX-PM (10%) in a syngeneic 4T1-luc breast tumor model based on measurements of tumor volume, 4T1-luc breast tumor bioluminescence, and survival. Importantly, intravenous administration of o(LA)8-PTX-PM is well tolerated by BALB/c mice. In summary, oligo(lactic acid)8-PTX is more compatible than PTX with PEG-b-PLA micelles, more stable, and may expand the role of PEG-b-PLA micelles from "solubilizer" into "nanocarrier" for PTX as a next-generation taxane for cancer.
Keywords: Block copolymer; Oligo(lactic acid); PEG; Polymeric micelle; Prodrug; Taxanes.
Copyright © 2019 Elsevier B.V. All rights reserved.
Figures



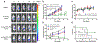

References
-
- McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, Clarke-Pearson DL, Davidson M, Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer, N. Engl. J. Med 334 (1996) 1–6. - PubMed
-
- Murphy WK, Fossella FV, Winn RJ, Shin DM, Hynes HE, Gross HM, Davilla E, Leimert J, Dhingra H, Raber MN, Krakoff IH, Phase II study of taxol in patients with untreated advanced non-small-cell lung cancer, J. Natl. Cancer Inst 85 (1993) 384–388. - PubMed
-
- Silvestris N, Galetta D, Colucci G, Successful treatment with three-weekly paclitaxel of an anthracycline-refractory classical Kaposi’s sarcoma, Anticancer Res. 29 (2009) 675–676. - PubMed
-
- Arnal I, Wade RH, How does taxol stabilize microtubules?, Curr. Biol 5 (1995) 900–908. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

