A deep learning approach for real-time detection of sleep spindles
- PMID: 30790769
- PMCID: PMC6527330
- DOI: 10.1088/1741-2552/ab0933
A deep learning approach for real-time detection of sleep spindles
Abstract
Objective: Sleep spindles have been implicated in memory consolidation and synaptic plasticity during NREM sleep. Detection accuracy and latency in automatic spindle detection are critical for real-time applications.
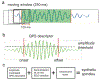
Approach: Here we propose a novel deep learning strategy (SpindleNet) to detect sleep spindles based on a single EEG channel. While the majority of spindle detection methods are used for off-line applications, our method is well suited for online applications.
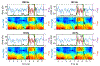
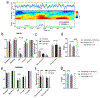
Main results: Compared with other spindle detection methods, SpindleNet achieves superior detection accuracy and speed, as demonstrated in two publicly available expert-validated EEG sleep spindle datasets. Our real-time detection of spindle onset achieves detection latencies of 150-350 ms (~two-three spindle cycles) and retains excellent performance under low EEG sampling frequencies and low signal-to-noise ratios. SpindleNet has good generalization across different sleep datasets from various subject groups of different ages and species.
Significance: SpindleNet is ultra-fast and scalable to multichannel EEG recordings, with an accuracy level comparable to human experts, making it appealing for long-term sleep monitoring and closed-loop neuroscience experiments.
Conflict of interest statement
Competing interests
Z.C., P.K., Z.X. have a pending US patent application. The remaining authors have no conflict of interest.
Figures
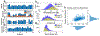




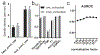
References
-
- Antoniades Andreas et al. 2017. “Detection of Interictal Discharges With Convolutional Neural Networks Using Discrete Ordered Multichannel Intracranial EEG.” IEEE Transactions on Neural Systems and Rehabilitation Engineering 25(12): 2285–94. - PubMed
-
- Astori Simone, Wimmer Ralf D., and Anita Lüthi. 2013. “Manipulating Sleep Spindles - Expanding Views on Sleep, Memory, and Disease.” Trends in Neurosciences 36(12): 738–48. - PubMed
-
- Babadi Behtash et al. 2012. “DiBa: A Data-Driven Bayesian Algorithm for Sleep Spindle Detection.” IEEE Transactions on Biomedical Engineering 59(2): 483–93. - PubMed
-
- Bashivan Pouya, Rish Irina, Yeasin Mohammed, and Codella Noel. 2015. “Learning Representations from EEG with Deep Recurrent-Convolutional Neural Networks.” : 1–15. http://arxiv.org/abs/1511.06448.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
