Cellular responses to ionizing radiation change quickly over time during early development in zebrafish
- PMID: 30791195
- PMCID: PMC6850130
- DOI: 10.1002/cbin.11117
Cellular responses to ionizing radiation change quickly over time during early development in zebrafish
Abstract
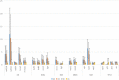
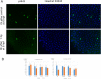
Animal cells constantly receive information about and respond to environmental factors, including ionizing radiation. Although it is crucial for a cell to repair radiation-induced DNA damage to ensure survival, cellular responses to radiation exposure during early embryonic development remain unclear. In this study, we analyzed the effects of ionizing radiation in zebrafish embryos and found that radiation-induced γH2AX foci formation and cell cycle arrest did not occur until the gastrula stage, despite the presence of major DNA repair-related gene transcripts, passed on as maternal factors. Interestingly, P21/WAF1 accumulation began ∼6 h post-fertilization, although p21 mRNA was upregulated by irradiation at 2 or 4 h post-fertilization. These results suggest that the cellular responses of zebrafish embryos at 2 or 4 h post-fertilization to radiation failed to overcome P21 protein accumulation and further signaling. Regulation of P21/WAF1 protein stabilization appears to be a key factor in the response to genotoxins during early embryogenesis.
Keywords: early development; radiation; zebrafish.
© 2019 The Authors. Cell Biology International Published by John Wiley & Sons Ltd on behalf of International Federation of Cell Biology.
Figures




References
-
- Adiga SK, Toyoshima M, Shimura T, Takeda J, Uematsu N, Niwa O (2007) Delayed and stage specific phosphorylation of H2AX during preimplantation development of γ‐irradiated mouse embryos. Reproduction 133(2): 415–22. - PubMed
-
- Brownlie A, Donovan A, Pratt SJ, Paw BH, Oates AC, Brugnara C, Witkowska HE, Sassa S, Zon LI (1998) Positional cloning of the zebrafish sauternes gene: a model for congenital sideroblastic anaemia. Nat Genet 20(3): 244–50. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

