Use of Titration as a Therapeutic Individualization Strategy: An Analysis of Food and Drug Administration-Approved Drugs
- PMID: 30791226
- PMCID: PMC6510374
- DOI: 10.1111/cts.12626
Use of Titration as a Therapeutic Individualization Strategy: An Analysis of Food and Drug Administration-Approved Drugs
Abstract
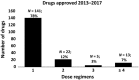
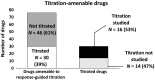
Selecting a dose regimen that is both safe and effective for patients is one of the most critical elements of a successful drug development program. Titrating the dose regimen of a drug based on patient response may help to identify safe and effective dosages at the individual patient level. Therefore, we quantified and characterized the use of response-guided titration for drugs recently approved by the US Food and Drug Administration (FDA) to assess how frequently this dosing strategy is used and how titration regimens are evaluated during drug development. Most of the 181 drugs approved from 2013-2017 (78%) had only one approved dosing regimen. Only 30 of 76 (39%) drugs that were considered amenable to response-guided dosing strategies had information in labeling about such strategies. These findings indicate that although response-guided titration can be found in labeling, this strategy is used in a minority of drugs for which it may be useful. Careful consideration should be made early in drug development as to whether a new drug is amenable to response-guided titration as an approach to reducing interpatient variability.
Published 2019. This article is a U.S. Government work and is in the public domain in the USA. Clinical and Translational Science published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures
References
-
- Cook, D. et al Lessons learned from the fate of Astrazeneca's drug pipeline: a five‐dimensional framework. Nat. Rev. Drug Discov. 13, 419–431 (2014). - PubMed
-
- Sacks, L.V. , Shamsuddin, H.H. , Yasinskaya, Y.I. , Bouri, K. , Lanthier, M.L. & Sherman, R.E. Scientific and regulatory reasons for delay and denial of FDA approval of initial applications for new drugs, 2000–2012. JAMA 311, 378–384 (2014). - PubMed
-
- Cook, J.C. , Wu, H. , Aleo, M.D. & Adkins, K. Principles of precision medicine and its application in toxicology. J. Toxicol. Sci. 43, 565–577 (2018). - PubMed
-
- International Conference on Harmonisation. Efficacy guideline E4: dose‐response information to support drug registration <http://www.ich.org/products/guidelines/efficacy/article/efficacy-guideli...> (1994).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources