Enhanced Anti-Allergic Activity of Milk Casein Phosphopeptide by Additional Phosphorylation in Ovalbumin-Sensitized Mice
- PMID: 30791382
- PMCID: PMC6412743
- DOI: 10.3390/molecules24040738
Enhanced Anti-Allergic Activity of Milk Casein Phosphopeptide by Additional Phosphorylation in Ovalbumin-Sensitized Mice
Abstract
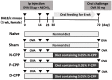
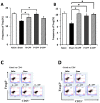
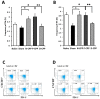
The proteolytic digest of milk casein, known as casein phosphopeptide (CPP-III), exhibits diverse biological activities, including calcium absorption and antioxidant activities. We hypothesized that the additional phosphorylation of this peptide can enhance its immunomodulatory activity such as suppression of allergy-associated cytokine and antigen-specific immune response. This study was conducted to assess whether oral intake of additionally phosphorylated CPP-III (P-CPP) attenuates ovalbumin (OVA)-induced IgE-mediated allergic reactions because of the additional phosphate groups. Female BALB/c mice were intraperitoneally sensitized with OVA twice at intervals of 14 days and then orally fed native CPP-III (N-CPP), P-CPP, and dephosphorylated CPP-III (D-CPP) for 6 weeks. Next, the mice were orally challenged with 50 mg of OVA. Oral administration of P-CPP suppressed total and specific IgE levels in the serum. Mice fed P-CPP exhibited low levels of OVA-specific IgG1 and increased OVA-specific IgG2a. P-CPP also suppressed IL-4 production, while D-CPP showed similar a level compared to that of the control. Further, P-CPP increased the population of the T follicular helper (Tfh) cell in the spleen. These results suggest that additional phosphorylation of CPP can enhance the attenuation of allergen-specific IgE-modulated allergic reactions in a murine food allergy model.
Keywords: anti-allergic activity; immunomodulation; phosphopeptide; phosphorylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Théolier J., Fliss I., Jean J., Hammami R. Antimicrobial Peptides of Dairy Proteins: From Fundamental to Applications. Food Rev. Int. 2014;30:134–154. doi: 10.1080/87559129.2014.896017. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

