Enhanced Ability of Oligomeric Nanobodies Targeting MERS Coronavirus Receptor-Binding Domain
- PMID: 30791410
- PMCID: PMC6410414
- DOI: 10.3390/v11020166
Enhanced Ability of Oligomeric Nanobodies Targeting MERS Coronavirus Receptor-Binding Domain
Abstract
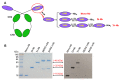
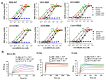
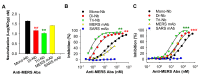
Middle East respiratory syndrome (MERS) coronavirus (MERS-CoV), an infectious coronavirus first reported in 2012, has a mortality rate greater than 35%. Therapeutic antibodies are key tools for preventing and treating MERS-CoV infection, but to date no such agents have been approved for treatment of this virus. Nanobodies (Nbs) are camelid heavy chain variable domains with properties distinct from those of conventional antibodies and antibody fragments. We generated two oligomeric Nbs by linking two or three monomeric Nbs (Mono-Nbs) targeting the MERS-CoV receptor-binding domain (RBD), and compared their RBD-binding affinity, RBD⁻receptor binding inhibition, stability, and neutralizing and cross-neutralizing activity against MERS-CoV. Relative to Mono-Nb, dimeric Nb (Di-Nb) and trimeric Nb (Tri-Nb) had significantly greater ability to bind MERS-CoV RBD proteins with or without mutations in the RBD, thereby potently blocking RBD⁻MERS-CoV receptor binding. The engineered oligomeric Nbs were very stable under extreme conditions, including low or high pH, protease (pepsin), chaotropic denaturant (urea), and high temperature. Importantly, Di-Nb and Tri-Nb exerted significantly elevated broad-spectrum neutralizing activity against at least 19 human and camel MERS-CoV strains isolated in different countries and years. Overall, the engineered Nbs could be developed into effective therapeutic agents for prevention and treatment of MERS-CoV infection.
Keywords: Coronavirus; MERS-CoV; cross-neutralization; nanobodies; receptor-binding domain; therapeutic antibodies.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
A Novel Nanobody Targeting Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Receptor-Binding Domain Has Potent Cross-Neutralizing Activity and Protective Efficacy against MERS-CoV.J Virol. 2018 Aug 29;92(18):e00837-18. doi: 10.1128/JVI.00837-18. Print 2018 Sep 15. J Virol. 2018. PMID: 29950421 Free PMC article.
-
Recombinant Receptor-Binding Domains of Multiple Middle East Respiratory Syndrome Coronaviruses (MERS-CoVs) Induce Cross-Neutralizing Antibodies against Divergent Human and Camel MERS-CoVs and Antibody Escape Mutants.J Virol. 2016 Dec 16;91(1):e01651-16. doi: 10.1128/JVI.01651-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795425 Free PMC article.
-
Single-dose treatment with a humanized neutralizing antibody affords full protection of a human transgenic mouse model from lethal Middle East respiratory syndrome (MERS)-coronavirus infection.Antiviral Res. 2016 Aug;132:141-8. doi: 10.1016/j.antiviral.2016.06.003. Epub 2016 Jun 14. Antiviral Res. 2016. PMID: 27312105 Free PMC article.
-
Advances in MERS-CoV Vaccines and Therapeutics Based on the Receptor-Binding Domain.Viruses. 2019 Jan 14;11(1):60. doi: 10.3390/v11010060. Viruses. 2019. PMID: 30646569 Free PMC article. Review.
-
Neutralizing Monoclonal Antibodies as Promising Therapeutics against Middle East Respiratory Syndrome Coronavirus Infection.Viruses. 2018 Nov 30;10(12):680. doi: 10.3390/v10120680. Viruses. 2018. PMID: 30513619 Free PMC article. Review.
Cited by
-
A potent SARS-CoV-2 neutralising nanobody shows therapeutic efficacy in the Syrian golden hamster model of COVID-19.Nat Commun. 2021 Sep 22;12(1):5469. doi: 10.1038/s41467-021-25480-z. Nat Commun. 2021. PMID: 34552091 Free PMC article.
-
COVID-19 and Hyperimmune sera: A feasible plan B to fight against coronavirus.Int Immunopharmacol. 2021 Jan;90:107220. doi: 10.1016/j.intimp.2020.107220. Epub 2020 Nov 20. Int Immunopharmacol. 2021. PMID: 33302034 Free PMC article. Review.
-
Monovalent, bivalent and biparatopic nanobodies targeting S1 protein of porcine epidemic diarrhea virus efficiently neutralized the virus infectivity.BMC Vet Res. 2024 Jul 30;20(1):336. doi: 10.1186/s12917-024-04151-3. BMC Vet Res. 2024. PMID: 39080763 Free PMC article.
-
Nanoscale warriors against viral invaders: a comprehensive review of Nanobodies as potential antiviral therapeutics.MAbs. 2025 Dec;17(1):2486390. doi: 10.1080/19420862.2025.2486390. Epub 2025 Apr 9. MAbs. 2025. PMID: 40201976 Free PMC article. Review.
-
Identification of Novel Natural Products as Effective and Broad-Spectrum Anti-Zika Virus Inhibitors.Viruses. 2019 Nov 2;11(11):1019. doi: 10.3390/v11111019. Viruses. 2019. PMID: 31684080 Free PMC article.
References
-
- Anthony S.J., Gilardi K., Menachery V.D., Goldstein T., Ssebide B., Mbabazi R., Navarrete-Macias I., Liang E., Wells H., Hicks A., et al. Further evidence for bats as the evolutionary source of Middle East respiratory syndrome coronavirus. MBio. 2017;8:e00373-17. doi: 10.1128/mBio.00373-17. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

