Genetic Evolution and Molecular Selection of the HE Gene of Influenza C Virus
- PMID: 30791465
- PMCID: PMC6409753
- DOI: 10.3390/v11020167
Genetic Evolution and Molecular Selection of the HE Gene of Influenza C Virus
Abstract
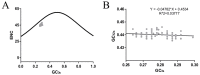
Influenza C virus (ICV) was first identified in humans and swine, but recently also in cattle, indicating a wider host range and potential threat to both the livestock industry and public health than was originally anticipated. The ICV hemagglutinin-esterase (HE) glycoprotein has multiple functions in the viral replication cycle and is the major determinant of antigenicity. Here, we developed a comparative approach integrating genetics, molecular selection analysis, and structural biology to identify the codon usage and adaptive evolution of ICV. We show that ICV can be classified into six lineages, consistent with previous studies. The HE gene has a low codon usage bias, which may facilitate ICV replication by reducing competition during evolution. Natural selection, dinucleotide composition, and mutation pressure shape the codon usage patterns of the ICV HE gene, with natural selection being the most important factor. Codon adaptation index (CAI) and relative codon deoptimization index (RCDI) analysis revealed that the greatest adaption of ICV was to humans, followed by cattle and swine. Additionally, similarity index (SiD) analysis revealed that swine exerted a stronger evolutionary pressure on ICV than humans, which is considered the primary reservoir. Furthermore, a similar tendency was also observed in the M gene. Of note, we found HE residues 176, 194, and 198 to be under positive selection, which may be the result of escape from antibody responses. Our study provides useful information on the genetic evolution of ICV from a new perspective that can help devise prevention and control strategies.
Keywords: Influenza C virus; codon usage bias; hemagglutinin-esterase fusion glycoprotein (HE); natural selection; selection pressure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




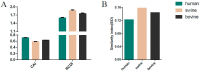

Similar articles
-
Evolutionary changes of the novel Influenza D virus hemagglutinin-esterase fusion gene revealed by the codon usage pattern.Virulence. 2019 Dec;10(1):1-9. doi: 10.1080/21505594.2018.1551708. Virulence. 2019. PMID: 30475085 Free PMC article.
-
Analyses of Evolutionary Characteristics of the Hemagglutinin-Esterase Gene of Influenza C Virus during a Period of 68 Years Reveals Evolutionary Patterns Different from Influenza A and B Viruses.Viruses. 2016 Nov 26;8(12):321. doi: 10.3390/v8120321. Viruses. 2016. PMID: 27898037 Free PMC article.
-
[Evolutionary analysis of the hemagglutinin-esterase (HE) gene of influenza C virus].Nihon Rinsho. 1997 Oct;55(10):2627-32. Nihon Rinsho. 1997. PMID: 9360382 Review. Japanese.
-
TMPRSS2 Activates Hemagglutinin-Esterase Glycoprotein of Influenza C Virus.J Virol. 2021 Oct 13;95(21):e0129621. doi: 10.1128/JVI.01296-21. Epub 2021 Aug 18. J Virol. 2021. PMID: 34406864 Free PMC article.
-
Structure and function of the HEF glycoprotein of influenza C virus.Adv Virus Res. 1991;40:213-34. doi: 10.1016/s0065-3527(08)60280-8. Adv Virus Res. 1991. PMID: 1957719 Free PMC article. Review.
Cited by
-
Competitive Cooperation of Hemagglutinin and Neuraminidase during Influenza A Virus Entry.Viruses. 2019 May 20;11(5):458. doi: 10.3390/v11050458. Viruses. 2019. PMID: 31137516 Free PMC article. Review.
-
Analysis of the Codon Usage Pattern of HA and NA Genes of H7N9 Influenza A Virus.Int J Mol Sci. 2020 Sep 27;21(19):7129. doi: 10.3390/ijms21197129. Int J Mol Sci. 2020. PMID: 32992529 Free PMC article.
-
Codon Usage in the Iflaviridae Family Is Not Diverse Though the Family Members Are Isolated from Diverse Host Taxa.Viruses. 2019 Nov 22;11(12):1087. doi: 10.3390/v11121087. Viruses. 2019. PMID: 31766648 Free PMC article.
-
Epidemiology and Clinical Characteristics of Influenza C Virus.Viruses. 2020 Jan 13;12(1):89. doi: 10.3390/v12010089. Viruses. 2020. PMID: 31941041 Free PMC article. Review.
-
Functional Characterization and Direct Comparison of Influenza A, B, C, and D NS1 Proteins in vitro and in vivo.Front Microbiol. 2019 Dec 17;10:2862. doi: 10.3389/fmicb.2019.02862. eCollection 2019. Front Microbiol. 2019. PMID: 31921042 Free PMC article.
References
-
- Guo Y.J., Jin F.G., Wang P., Wang M., Zhu J.M. Isolation of influenza c virus from pigs and experimental infection of pigs with influenza c virus. J. Gen. Virol. 1983;64:177–182. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

