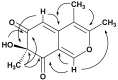
Chlamyphilone, a Novel Pochonia chlamydosporia Metabolite with Insecticidal Activity
- PMID: 30791467
- PMCID: PMC6412625
- DOI: 10.3390/molecules24040750
Chlamyphilone, a Novel Pochonia chlamydosporia Metabolite with Insecticidal Activity
Abstract
Metabolites from a collection of selected fungal isolates have been screened for insecticidal activity against the aphid Acyrthosiphon pisum. Crude organic extracts of culture filtrates from six fungal isolates (Paecilomyces lilacinus, Pochonia chlamydosporia, Penicillium griseofulvum, Beauveria bassiana, Metarhizium anisopliae and Talaromyces pinophilus) caused mortality of aphids within 72 h after treatment. In this work, bioassay-guided fractionation has been used to characterize the main bioactive metabolites accumulated in fungal extracts. Leucinostatins A, B and D represent the bioactive compounds produced by P. lilacinus. From P. griseofulvum and B. bassiana extracts, griseofulvin and beauvericin have been isolated, respectively; 3-O-Methylfunicone and a mixture of destruxins have been found in the active fractions of T. pinophilum and M. anisopliae, respectively. A novel azaphilone compound, we named chlamyphilone, with significant insecticidal activity, has been isolated from the culture filtrate of P. chlamydosporia. Its structure has been determined using extensive spectroscopic methods and chemical derivatization.
Keywords: azaphilones; beneficial microbes; pea aphid; secondary metabolites.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources