Potential of Nanomaterial Applications in Dietary Supplements and Foods for Special Medical Purposes
- PMID: 30791492
- PMCID: PMC6409737
- DOI: 10.3390/nano9020296
Potential of Nanomaterial Applications in Dietary Supplements and Foods for Special Medical Purposes
Abstract
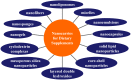
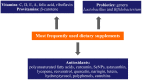
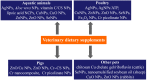
Dietary supplements and foods for special medical purposes are special medical products classified according to the legal basis. They are regulated, for example, by the European Food Safety Authority and the U.S. Food and Drug Administration, as well as by various national regulations issued most frequently by the Ministry of Health and/or the Ministry of Agriculture of particular countries around the world. They constitute a concentrated source of vitamins, minerals, polyunsaturated fatty acids and antioxidants or other compounds with a nutritional or physiological effect contained in the food/feed, alone or in combination, intended for direct consumption in small measured amounts. As nanotechnology provides "a new dimension" accompanied with new or modified properties conferred to many current materials, it is widely used for the production of a new generation of drug formulations, and it is also used in the food industry and even in various types of nutritional supplements. These nanoformulations of supplements are being prepared especially with the purpose to improve bioavailability, protect active ingredients against degradation, or reduce side effects. This contribution comprehensively summarizes the current state of the research focused on nanoformulated human and veterinary dietary supplements, nutraceuticals, and functional foods for special medical purposes, their particular applications in various food products and drinks as well as the most important related guidelines, regulations and directives.
Keywords: bioactive agents; dietary supplements; encapsulation; feed; foodstuffs; nanoemulsions; nanoformulations; nanoparticles; nutraceuticals.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Rao P.J., Naidu M.M. Nanoencapsulation of Bioactive Compounds for Nutraceutical Food. In: Ranjan S., Dasgupta N., Lichtfouse E., editors. Nanoscience in Food and Agriculture 2. Sustainable Agriculture Reviews. Volume 21. Springer; Cham, Germany: 2016. pp. 129–156.
-
- National Institutes of Health Dietary Supplements: Background Information. [(accessed on 20 November 2018)]; Available online: https://ods.od.nih.gov/factsheets/DietarySupplements-HealthProfessional/
-
- European Commission Food Supplements. [(accessed on 20 November 2018)]; Available online: https://ec.europa.eu/food/safety/labelling_nutrition/supplements_en.
-
- U.S. Food and Drug Administration Dietary Supplements Guidance Documents & Regulatory Information. [(accessed on 20 November 2018)]; Available online: https://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryI....
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials