Autophagy as a Therapeutic Target to Enhance Aged Muscle Regeneration
- PMID: 30791569
- PMCID: PMC6406986
- DOI: 10.3390/cells8020183
Autophagy as a Therapeutic Target to Enhance Aged Muscle Regeneration
Abstract
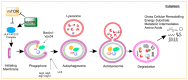
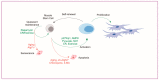
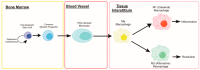
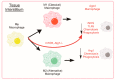
Skeletal muscle has remarkable regenerative capacity, relying on precise coordination between resident muscle stem cells (satellite cells) and the immune system. The age-related decline in skeletal muscle regenerative capacity contributes to the onset of sarcopenia, prolonged hospitalization, and loss of autonomy. Although several age-sensitive pathways have been identified, further investigation is needed to define targets of cellular dysfunction. Autophagy, a process of cellular catabolism, is emerging as a key regulator of muscle regeneration affecting stem cell, immune cell, and myofiber function. Muscle stem cell senescence is associated with a suppression of autophagy during key phases of the regenerative program. Macrophages, a key immune cell involved in muscle repair, also rely on autophagy to aid in tissue repair. This review will focus on the role of autophagy in various aspects of the regenerative program, including adult skeletal muscle stem cells, monocytes/macrophages, and corresponding age-associated dysfunction. Furthermore, we will highlight rejuvenation strategies that alter autophagy to improve muscle regenerative function.
Keywords: aging; caloric restriction; exercise; immune; macrophage; muscle regeneration; senescence; stem cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Understanding muscle regenerative decline with aging: new approaches to bring back youthfulness to aged stem cells.FEBS J. 2020 Feb;287(3):406-416. doi: 10.1111/febs.15182. Epub 2020 Jan 3. FEBS J. 2020. PMID: 31854082 Review.
-
Dysfunctional autophagy is a driver of muscle stem cell functional decline with aging.Autophagy. 2016;12(3):612-3. doi: 10.1080/15548627.2016.1143211. Autophagy. 2016. PMID: 26890313 Free PMC article.
-
Regenerative decline of stem cells in sarcopenia.Mol Aspects Med. 2016 Aug;50:109-17. doi: 10.1016/j.mam.2016.02.002. Epub 2016 Feb 24. Mol Aspects Med. 2016. PMID: 26921790 Review.
-
Regenerative function of immune system: Modulation of muscle stem cells.Ageing Res Rev. 2016 May;27:67-76. doi: 10.1016/j.arr.2016.03.006. Epub 2016 Mar 31. Ageing Res Rev. 2016. PMID: 27039885 Review.
-
Muscle stem cell aging: identifying ways to induce tissue rejuvenation.Mech Ageing Dev. 2020 Jun;188:111246. doi: 10.1016/j.mad.2020.111246. Epub 2020 Apr 18. Mech Ageing Dev. 2020. PMID: 32311419
Cited by
-
The Effects of Calorie Restriction on Autophagy: Role on Aging Intervention.Nutrients. 2019 Dec 2;11(12):2923. doi: 10.3390/nu11122923. Nutrients. 2019. PMID: 31810345 Free PMC article. Review.
-
Differential Autophagy Response in Men and Women After Muscle Damage.Front Physiol. 2021 Nov 24;12:752347. doi: 10.3389/fphys.2021.752347. eCollection 2021. Front Physiol. 2021. PMID: 34899384 Free PMC article.
-
The role and therapeutic potential of stem cells in skeletal muscle in sarcopenia.Stem Cell Res Ther. 2022 Jan 24;13(1):28. doi: 10.1186/s13287-022-02706-5. Stem Cell Res Ther. 2022. PMID: 35073997 Free PMC article. Review.
-
Autophagy mediated by ROS-AKT-FoxO pathway is required for intestinal regeneration in echinoderms.Cell Commun Signal. 2025 Jan 7;23(1):8. doi: 10.1186/s12964-024-01993-0. Cell Commun Signal. 2025. PMID: 39762855 Free PMC article.
-
In vivo monitoring of tissue regeneration using a ratiometric lysosomal AIE probe.Chem Sci. 2020 Feb 11;11(12):3152-3163. doi: 10.1039/c9sc06226b. Chem Sci. 2020. PMID: 34122820 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

