Fusarisolins A⁻E, Polyketides from the Marine-Derived Fungus Fusarium solani H918
- PMID: 30791608
- PMCID: PMC6410219
- DOI: 10.3390/md17020125
Fusarisolins A⁻E, Polyketides from the Marine-Derived Fungus Fusarium solani H918
Abstract
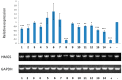
Five new (fusarisolins A⁻E, 1 to 5) and three known (6 to 8) polyketides were isolated from the marine-derived fungus Fusarium solani H918, along with six known phenolics (9 to 14). Their structures were established by comprehensive spectroscopic data analyses, methoxyphenylacetic acid (MPA) method, chemical conversion, and by comparison with data reported in the literature. Compounds 1 and 2 are the first two naturally occurring 21 carbons polyketides featuring a rare β- and γ-lactone unit, respectively. All isolates (1 to 14) were evaluated for their inhibitory effects against tea pathogenic fungus Pestalotiopsis theae and 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) synthase gene expression. Compound 8 showed potent antifungal activity with an ED50 value of 55 μM, while 1, 8, 13, and 14 significantly inhibited HMG-CoA synthase gene expression.
Keywords: HMG-CoA synthase; bioactivity; microorganisms; ocean; β-lactones.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

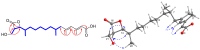
 ), HMBC (
), HMBC ( ), and NOESY (
), and NOESY ( ) correlations of 1.
) correlations of 1.
References
-
- Greenspan M.D., Yudkovitz J.B., Lo C.Y.L., Chen J.S., Alberts A.W., Hunt V.M., Chang M.N., Yang S.S., Thompson K.L., Chiang Y.C.P., et al. Inhibition of hydroxymethylglutaryl-coenzyme A synthase by L-659,699. Proc. Natl. Acad. Sci. USA. 1987;84:7488–7492. doi: 10.1073/pnas.84.21.7488. - DOI - PMC - PubMed
-
- Tomoda H., Kumagai H., Takahashi Y., Tanaka Y., Iwai Y., Omura S. F-244 (1233A), a specific inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A synthase: Taxonomy of producing strain, fermentation, isolation and biological properties. J. Antibiot. 1988;41:247–249. doi: 10.7164/antibiotics.41.247. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 2017035/Scientific Research Foundation of Third Institute of Oceanography, SOA
- 2018009/Scientific Research Foundation of Third Institute of Oceanography, SOA
- 41606185/National Natural Science Foundation of China
- 41676130/National Natural Science Foundation of China
- 3502Z20172009/Xiamen Science and Technology Program
LinkOut - more resources
Full Text Sources

