Regulator of G-Protein Signaling 16 Is a Negative Modulator of Platelet Function and Thrombosis
- PMID: 30791801
- PMCID: PMC6474914
- DOI: 10.1161/JAHA.118.011273
Regulator of G-Protein Signaling 16 Is a Negative Modulator of Platelet Function and Thrombosis
Abstract
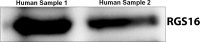
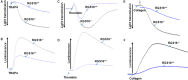
Background Members of the regulator of G-protein signaling ( RGS ) family inhibit G-protein coupled receptor signaling by modulating G-protein activity. In platelets, there are 3 different RGS isoforms that are expressed at the protein level, including RGS 16. Recently, we have shown that CXCL 12 regulates platelet function via RGS 16. However, the role of RGS 16 in platelet function and thrombus formation is poorly defined. Methods and Results We used a genetic knockout mouse model approach to examine the role(s) of RGS 16 in platelet activation by using a host of in vitro and in vivo assays. We observed that agonist-induced platelet aggregation, secretion, and integrin activation were much more pronounced in platelets from the RGS 16 knockout ( Rgs16 -/-) mice relative to their wild type ( Rgs16 +/+) littermates. Furthermore, the Rgs16 -/- mice had a markedly shortened bleeding time and were more susceptible to vascular injury-associated thrombus formation than the controls. Conclusions These findings support a critical role for RGS 16 in regulating hemostatic and thrombotic functions of platelets in mice. Hence, RGS 16 represents a potential therapeutic target for modulating platelet function.
Keywords: RGS16; hemostasis; platelet; regulator of G‐protein signaling; signal transduction; thrombosis.
Figures

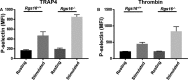
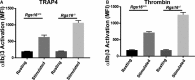
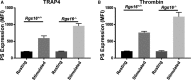

References
-
- Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–949. - PubMed
-
- Jackson SP. Arterial thrombosis—insidious, unpredictable and deadly. Nat Med. 2011;17:1423–1436. - PubMed
-
- Nieswandt B, Pleines I, Bender M. Platelet adhesion and activation mechanisms in arterial thrombosis and ischaemic stroke. J Thromb Haemost. 2011;9(suppl 1):92–104. - PubMed
-
- Wiisanen ME, Moliterno DJ. Platelet protease‐activated receptor antagonism in cardiovascular medicine. Coron Artery Dis. 2012;23:375–379. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

