Dose-painting multicenter phase III trial in newly diagnosed glioblastoma: the SPECTRO-GLIO trial comparing arm A standard radiochemotherapy to arm B radiochemotherapy with simultaneous integrated boost guided by MR spectroscopic imaging
- PMID: 30791889
- PMCID: PMC6385401
- DOI: 10.1186/s12885-019-5317-x
Dose-painting multicenter phase III trial in newly diagnosed glioblastoma: the SPECTRO-GLIO trial comparing arm A standard radiochemotherapy to arm B radiochemotherapy with simultaneous integrated boost guided by MR spectroscopic imaging
Abstract
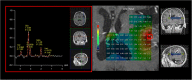
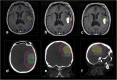
Background: Glioblastoma, a high-grade glial infiltrating tumor, is the most frequent malignant brain tumor in adults and carries a dismal prognosis. External beam radiotherapy (EBRT) increases overall survival but this is still low due to local relapses, mostly occurring in the irradiation field. As the ratio of spectra of choline/N acetyl aspartate> 2 (CNR2) on MR spectroscopic imaging has been described as predictive for the site of local relapse, we hypothesized that dose escalation on these regions would increase local control and hence global survival.
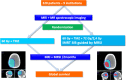
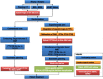
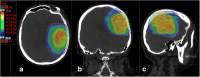
Methods/design: In this multicenter prospective phase III trial for newly diagnosed glioblastoma, 220 patients having undergone biopsy or surgery are planned for randomization to two arms. Arm A is the Stupp protocol (EBRT 60 Gy on contrast enhancement + 2 cm margin with concomitant temozolomide (TMZ) and 6 months of TMZ maintenance); Arm B is the same treatment with an additional simultaneous integrated boost of intensity-modulated radiotherapy (IMRT) of 72Gy/2.4Gy delivered on the MR spectroscopic imaging metabolic volumes of CHO/NAA > 2 and contrast-enhancing lesions or resection cavity. Stratification is performed on surgical and MGMT status.
Discussion: This is a dose-painting trial, i.e. delivery of heterogeneous dose guided by metabolic imaging. The principal endpoint is overall survival. An online prospective quality control of volumes and dose is performed in the experimental arm. The study will yield a large amount of longitudinal multimodal MR imaging data including planning CT, radiotherapy dosimetry, MR spectroscopic, diffusion and perfusion imaging.
Trial registration: NCT01507506 , registration date December 20, 2011.
Keywords: Clinical trial; Dose-painting; Glioblastoma; Magnetic resonance spectroscopic imaging; Phase III,online prospective quality control; Proton spectroscopy; Radiotherapy; Spectroscopy.
Conflict of interest statement
Ethics approval and consent to participate
This trial was reviewed and approved by the French ethics committee on 28 April 2010: registration number 2009-A00594–53.
This covers all participating centers.which are:
Institut Claudius Regaud at Institut Universitaire du Cancer de Toulouse-Oncopole- Toulouse-France.
Centre Paul Strauss- Strasbourg- France.
Centre Georges-François Leclerc – Dijon- France.
Centre Léon Bérard- Lyon- France.
Institut de Cancérologie de la Loire- Saint-Priest en Jarez.
Centre Val d’aurelle – Montpellier- France.
Clinique Claude Bernard- Albi- France.
All subjects signed a written informed consent form.
Consent for publication
Consent for publication is not applicable to this article.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Laprie A, Catalaa I, Cassol E, McKnight TR, Berchery D, Marre D, et al. Proton magnetic resonance spectroscopic imaging in newly diagnosed glioblastoma: predictive value for the site of Postradiotherapy relapse in a prospective longitudinal study. Int J Radiat Oncol Biol Phys. 2008;70:773–781. doi: 10.1016/j.ijrobp.2007.10.039. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

