Structure-activity relationships for unit C pyridyl analogues of the tuberculosis drug bedaquiline
- PMID: 30792104
- PMCID: PMC6467542
- DOI: 10.1016/j.bmc.2019.02.025
Structure-activity relationships for unit C pyridyl analogues of the tuberculosis drug bedaquiline
Abstract
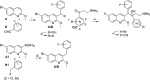
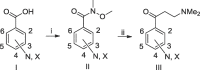
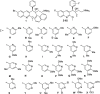
The ATP-synthase inhibitor bedaquiline is effective against drug-resistant tuberculosis but is extremely lipophilic (clogP 7.25) with a very long plasma half-life. Additionally, inhibition of potassium current through the cardiac hERG channel by bedaquiline, is associated with prolongation of the QT interval, necessitating cardiovascular monitoring. Analogues were prepared where the naphthalene C-unit was replaced with substituted pyridines to produce compounds with reduced lipophilicity, anticipating a reduction in half-life. While there was a direct correlation between in vitro inhibitory activity against M. tuberculosis (MIC90) and compound lipophilicity, potency only fell off sharply below a clogP of about 4.0, providing a useful lower bound for analogue design. The bulk of the compounds remained potent inhibitors of the hERG potassium channel, with notable exceptions where IC50 values were at least 5-fold higher than that of bedaquiline. Many of the compounds had desirably higher rates of clearance than bedaquiline, but this was associated with lower plasma exposures in mice, and similar or higher MICs resulted in lower AUC/MIC ratios than bedaquiline for most compounds. The two compounds with lower potency against hERG exhibited similar clearance to bedaquiline and excellent efficacy in vivo, suggesting further exploration of C-ring pyridyls is worthwhile.
Keywords: Bedaquiline; Bedaquiline analogues; Drug development; Lipophilicity; Tuberculosis.
Copyright © 2019 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
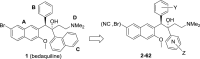
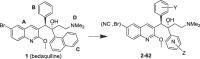
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Chemical Information

