Neuron-Specific Genome Modification in the Adult Rat Brain Using CRISPR-Cas9 Transgenic Rats
- PMID: 30792150
- PMCID: PMC11805586
- DOI: 10.1016/j.neuron.2019.01.035
Neuron-Specific Genome Modification in the Adult Rat Brain Using CRISPR-Cas9 Transgenic Rats
Abstract
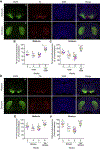
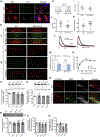
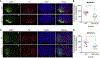
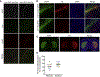
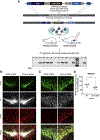
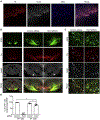
Historically, the rat has been the preferred animal model for behavioral studies. Limitations in genome modification have, however, caused a lag in their use compared to the bevy of available transgenic mice. Here, we have developed several transgenic tools, including viral vectors and transgenic rats, for targeted genome modification in specific adult rat neurons using CRISPR-Cas9 technology. Starting from wild-type rats, knockout of tyrosine hydroxylase was achieved with adeno-associated viral (AAV) vectors expressing Cas9 or guide RNAs (gRNAs). We subsequently created an AAV vector for Cre-dependent gRNA expression as well as three new transgenic rat lines to specifically target CRISPR-Cas9 components to dopaminergic neurons. One rat represents the first knockin rat model made by germline gene targeting in spermatogonial stem cells. The rats described herein serve as a versatile platform for making cell-specific and sequence-specific genome modifications in the adult brain and potentially other Cre-expressing tissues of the rat.
Keywords: AAV; CRISPR-Cas9; MANF; brain; genome editing; lsl-Cas9; spermatogonial stem cells; transgenic rat.
Published by Elsevier Inc.
Figures


References
-
- Bae S, Kweon J, Kim HS, and Kim JS (2014). Microhomology-based choice of Cas9 nuclease target sites. Nat. Methods 11, 705–706. - PubMed
-
- Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, and Horvath P. (2007). CRISPR provides acquired resis- tance against viruses in prokaryotes. Science 315, 1709–1712. - PubMed
-
- Cho SW, Kim S, Kim JM, and Kim JS (2013). Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat. Biotechnol. 31, 230–232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

