Reactive species and pathogen antioxidant networks during phagocytosis
- PMID: 30792185
- PMCID: PMC6400530
- DOI: 10.1084/jem.20181886
Reactive species and pathogen antioxidant networks during phagocytosis
Abstract
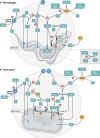
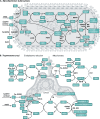
The generation of phagosomal cytotoxic reactive species (i.e., free radicals and oxidants) by activated macrophages and neutrophils is a crucial process for the control of intracellular pathogens. The chemical nature of these species, the reactions they are involved in, and the subsequent effects are multifaceted and depend on several host- and pathogen-derived factors that influence their production rates and catabolism inside the phagosome. Pathogens rely on an intricate and synergistic antioxidant armamentarium that ensures their own survival by detoxifying reactive species. In this review, we discuss the generation, kinetics, and toxicity of reactive species generated in phagocytes, with a focus on the response of macrophages to internalized pathogens and concentrating on Mycobacterium tuberculosis and Trypanosoma cruzi as examples of bacterial and parasitic infection, respectively. The ability of pathogens to deal with host-derived reactive species largely depends on the competence of their antioxidant networks at the onset of invasion, which in turn can tilt the balance toward pathogen survival, proliferation, and virulence over redox-dependent control of infection.
© 2019 Piacenza et al.
Figures
References
-
- Albesa-Jové D., Chiarelli L.R., Makarov V., Pasca M.R., Urresti S., Mori G., Salina E., Vocat A., Comino N., Mohorko E., et al. 2014. Rv2466c mediates the activation of TP053 to kill replicating and non-replicating Mycobacterium tuberculosis. ACS Chem. Biol. 9:1567–1575. 10.1021/cb500149m - DOI - PubMed
-
- Alegria T.G., Meireles D.A., Cussiol J.R., Hugo M., Trujillo M., de Oliveira M.A., Miyamoto S., Queiroz R.F., Valadares N.F., Garratt R.C., et al. 2017. Ohr plays a central role in bacterial responses against fatty acid hydroperoxides and peroxynitrite. Proc. Natl. Acad. Sci. USA. 114:E132–E141. 10.1073/pnas.1619659114 - DOI - PMC - PubMed
-
- Allmann S., Morand P., Ebikeme C., Gales L., Biran M., Hubert J., Brennand A., Mazet M., Franconi J.M., Michels P.A., et al. 2013. Cytosolic NADPH homeostasis in glucose-starved procyclic Trypanosoma brucei relies on malic enzyme and the pentose phosphate pathway fed by gluconeogenic flux. J. Biol. Chem. 288:18494–18505. 10.1074/jbc.M113.462978 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

