The Strength of Alpha-Beta Oscillatory Coupling Predicts Motor Timing Precision
- PMID: 30792271
- PMCID: PMC6788828
- DOI: 10.1523/JNEUROSCI.2473-18.2018
The Strength of Alpha-Beta Oscillatory Coupling Predicts Motor Timing Precision
Abstract
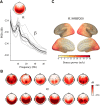
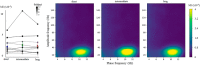
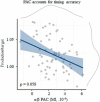
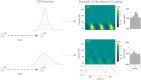
Precise timing makes the difference between harmony and cacophony, but how the brain achieves precision during timing is unknown. In this study, human participants (7 females, 5 males) generated a time interval while being recorded with magnetoencephalography. Building on the proposal that the coupling of neural oscillations provides a temporal code for information processing in the brain, we tested whether the strength of oscillatory coupling was sensitive to self-generated temporal precision. On a per individual basis, we show the presence of alpha-beta phase-amplitude coupling whose strength was associated with the temporal precision of self-generated time intervals, not with their absolute duration. Our results provide evidence that active oscillatory coupling engages α oscillations in maintaining the precision of an endogenous temporal motor goal encoded in β power; the when of self-timed actions. We propose that oscillatory coupling indexes the variance of neuronal computations, which translates into the precision of an individual's behavioral performance.SIGNIFICANCE STATEMENT Which neural mechanisms enable precise volitional timing in the brain is unknown, yet accurate and precise timing is essential in every realm of life. In this study, we build on the hypothesis that neural oscillations, and their coupling across time scales, are essential for the coding and for the transmission of information in the brain. We show the presence of alpha-beta phase-amplitude coupling (α-β PAC) whose strength was associated with the temporal precision of self-generated time intervals, not with their absolute duration. α-β PAC indexes the temporal precision with which information is represented in an individual's brain. Our results link large-scale neuronal variability on the one hand, and individuals' timing precision, on the other.
Keywords: alpha; beta; cross-frequency coupling; phase–amplitude coupling; time perception; timing.
Copyright © 2019 the authors.
Figures








References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
