Latrophilin GPCRs direct synapse specificity by coincident binding of FLRTs and teneurins
- PMID: 30792275
- PMCID: PMC6636343
- DOI: 10.1126/science.aav7969
Latrophilin GPCRs direct synapse specificity by coincident binding of FLRTs and teneurins
Erratum in
-
Erratum for the Research Article "Latrophilin GPCRs direct synapse specificity by coincident binding of FLRTs and teneurins" by R. Sando et al.Science. 2024 May 3;384(6695):eadp9617. doi: 10.1126/science.adp9617. Epub 2024 May 2. Science. 2024. PMID: 38696585 No abstract available.
Abstract
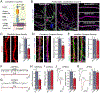
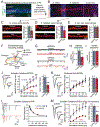
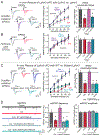
Bidirectional signaling by cell adhesion molecules is thought to mediate synapse formation, but the mechanisms involved remain elusive. We found that the adhesion G protein-coupled receptors latrophilin-2 and latrophilin-3 selectively direct formation of perforant-path and Schaffer-collateral synapses, respectively, to hippocampal CA1-region neurons. Latrophilin-3 binds to two transcellular ligands: fibronectin leucine-rich repeat transmembrane proteins (FLRTs) and teneurins. In transgenic mice in vivo, both binding activities were required for input-specific synapse formation, which suggests that coincident binding of both ligands is necessary for synapse formation. In cultured neurons in vitro, teneurin or FLRT alone did not induce excitatory synapse formation, whereas together they potently did so. Thus, postsynaptic latrophilins promote excitatory synapse formation by simultaneous binding of two unrelated presynaptic ligands, which is required for formation of synaptic inputs at specific dendritic localizations.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures




References
-
- Sanes JR, Yamagata M, Many paths to synaptic specificity. Annu. Rev. Cell Dev. Biol. 25, 161–195 (2009). - PubMed
-
- Nusser Z, Creating diverse synapses from the same molecules. Curr. Opin. Neurobiol. 51, 8–15 (2018). - PubMed
-
- Silva JP, Lelianova VG, Ermolyuk YS, Vysokov N, Hitchen PG, Berninghausen O, Rahman MA, Zangrandi A, Fidalgo S, Tonevitsky AG, Dell A, Volynski KE, Ushkaryov YA, Latrophilin 1 and its endogenous ligand Lasso/teneurin-2 form a high-affinity transsynaptic receptor pair with signaling capabilities. Proc. Natl. Acad. Sci. USA. 108, 12113–12118 (2011). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

