Catalytic reductive [4 + 1]-cycloadditions of vinylidenes and dienes
- PMID: 30792299
- PMCID: PMC6467289
- DOI: 10.1126/science.aau0364
Catalytic reductive [4 + 1]-cycloadditions of vinylidenes and dienes
Abstract
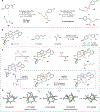
Cycloaddition reactions provide direct and convergent routes to cycloalkanes, making them valuable targets for the development of synthetic methods. Whereas six-membered rings are readily accessible from Diels-Alder reactions, cycloadditions that generate five-membered rings are comparatively limited in scope. Here, we report that dinickel complexes catalyze [4 + 1]-cycloaddition reactions of 1,3-dienes. The C1 partner is a vinylidene equivalent generated from the reductive activation of a 1,1-dichloroalkene in the presence of stoichiometric zinc. Intermolecular and intramolecular variants of the reaction are described, and high levels of asymmetric induction are achieved in the intramolecular cycloadditions using a C 2-symmetric chiral ligand that stabilizes a metal-metal bond.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures



Comment in
-
When two metal atoms are better than one.Science. 2019 Feb 22;363(6429):819. doi: 10.1126/science.aaw5825. Science. 2019. PMID: 30792292 No abstract available.
References
-
- Trost BM, [3+2] Cycloaddition approaches to five-membered rings via trimethylenemethane and its equivalents [New Synthetic Methods (55)]. Angew. Chem., Int. Ed 25, 1–20 (1986); doi: 10.1002/anie.198600013 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

