Mitochondrial localization, import, and mitochondrial function of the androgen receptor
- PMID: 30792308
- PMCID: PMC6484137
- DOI: 10.1074/jbc.RA118.006727
Mitochondrial localization, import, and mitochondrial function of the androgen receptor
Abstract
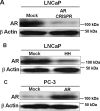
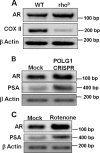
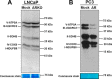
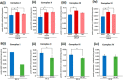
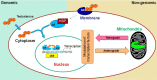
Nuclear localization of androgen receptor (AR) directs transcriptional regulation of a host of genes, referred to as genomic signaling. Additionally, nonnuclear or nongenomic activities of the AR have long been described, but understanding of these activities remains elusive. Here, we report that AR is imported into and localizes to mitochondria and has a novel role in regulating multiple mitochondrial processes. Employing complementary experimental approaches of AR knockdown in AR-expressing cells and ectopic AR expression in AR-deficient cells, we demonstrate an inverse relationship between AR expression and mitochondrial DNA (mtDNA) content and transcription factor A, mitochondrial (TFAM), a regulator of mtDNA content. We show that AR localizes to mitochondria in prostate tissues and cell lines and is imported into mitochondria in vitro We also found that AR contains a 36-amino-acid-long mitochondrial localization sequence (MLS) capable of targeting a passenger protein (GFP) to the mitochondria and that deletion of the MLS abolishes the import of AR into the mitochondria. Ectopic AR expression reduced the expression of oxidative phosphorylation (OXPHOS) subunits. Interestingly, AR also controlled translation of mtDNA-encoded genes by regulating expression of multiple nuclear DNA-encoded mitochondrial ribosomal proteins. Consistent with these observations, OXPHOS supercomplexes were destabilized, and OXPHOS enzymatic activities were reduced in AR-expressing cells and restored upon AR knockdown. Moreover, mitochondrial impairment induced AR expression and increased its translocation into mitochondria. We conclude that AR localizes to mitochondria, where it controls multiple mitochondrial functions and mitonuclear communication. Our studies also suggest that mitochondria are novel players in nongenomic activities of AR.
Keywords: androgen receptor; castration-resistant; cell signaling; gene transcription; genomic signaling; mitochondria; mitochondrial localization sequence; nongenomic signaling; nuclear receptor; oxidative phosphorylation; prostate cancer; retrograde signaling.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures






Similar articles
-
Androgen-induced expression of DRP1 regulates mitochondrial metabolic reprogramming in prostate cancer.Cancer Lett. 2020 Feb 28;471:72-87. doi: 10.1016/j.canlet.2019.12.017. Epub 2019 Dec 12. Cancer Lett. 2020. PMID: 31838085
-
Suppression of Mic60 compromises mitochondrial transcription and oxidative phosphorylation.Sci Rep. 2015 Jan 23;5:7990. doi: 10.1038/srep07990. Sci Rep. 2015. PMID: 25612828 Free PMC article.
-
A novel androgen receptor antagonist JJ-450 inhibits enzalutamide-resistant mutant ARF876L nuclear import and function.Prostate. 2020 Mar;80(4):319-328. doi: 10.1002/pros.23945. Epub 2019 Dec 23. Prostate. 2020. PMID: 31868960 Free PMC article.
-
Mitochondrial transcription: lessons from mouse models.Biochim Biophys Acta. 2012 Sep-Oct;1819(9-10):961-9. doi: 10.1016/j.bbagrm.2011.11.001. Epub 2011 Nov 18. Biochim Biophys Acta. 2012. PMID: 22120174 Free PMC article. Review.
-
Mitochondrial transcription factor A regulates mitochondrial transcription initiation, DNA packaging, and genome copy number.Biochim Biophys Acta. 2012 Sep-Oct;1819(9-10):921-9. doi: 10.1016/j.bbagrm.2012.03.002. Epub 2012 Mar 21. Biochim Biophys Acta. 2012. PMID: 22465614 Review.
Cited by
-
Peripheral Blood Cells From Older Adults Exhibit Sex-Associated Differences in Mitochondrial Function.J Gerontol A Biol Sci Med Sci. 2024 May 1;79(5):glae098. doi: 10.1093/gerona/glae098. J Gerontol A Biol Sci Med Sci. 2024. PMID: 38602189 Free PMC article.
-
From mitochondria to sarcopenia: role of 17β-estradiol and testosterone.Front Endocrinol (Lausanne). 2023 Apr 20;14:1156583. doi: 10.3389/fendo.2023.1156583. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37152937 Free PMC article. Review.
-
The Roles of Androgens in Humans: Biology, Metabolic Regulation and Health.Int J Mol Sci. 2022 Oct 8;23(19):11952. doi: 10.3390/ijms231911952. Int J Mol Sci. 2022. PMID: 36233256 Free PMC article. Review.
-
Advances in mitochondria-centered mechanism behind the roles of androgens and androgen receptor in the regulation of glucose and lipid metabolism.Front Endocrinol (Lausanne). 2023 Oct 13;14:1267170. doi: 10.3389/fendo.2023.1267170. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37900128 Free PMC article. Review.
-
Increased uterine androgen receptor protein abundance results in implantation and mitochondrial defects in pregnant rats with hyperandrogenism and insulin resistance.J Mol Med (Berl). 2021 Oct;99(10):1427-1446. doi: 10.1007/s00109-021-02104-z. Epub 2021 Jun 28. J Mol Med (Berl). 2021. PMID: 34180022 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

