Similarities between action potentials and acoustic pulses in a van der Waals fluid
- PMID: 30792493
- PMCID: PMC6385226
- DOI: 10.1038/s41598-019-38826-x
Similarities between action potentials and acoustic pulses in a van der Waals fluid
Abstract
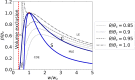
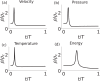
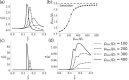
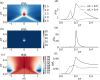
An action potential is typically described as a purely electrical change that propagates along the membrane of excitable cells. However, recent experiments have demonstrated that non-linear acoustic pulses that propagate along lipid interfaces and traverse the melting transition, share many similar properties with action potentials. Despite the striking experimental similarities, a comprehensive theoretical study of acoustic pulses in lipid systems is still lacking. Here we demonstrate that an idealized description of an interface near phase transition captures many properties of acoustic pulses in lipid monolayers, as well as action potentials in living cells. The possibility that action potentials may better be described as acoustic pulses in soft interfaces near phase transition is illustrated by the following similar properties: correspondence of time and velocity scales, qualitative pulse shape, sigmoidal response to stimulation amplitude (an 'all-or-none' behavior), appearance in multiple observables (particularly, an adiabatic change of temperature), excitation by many types of stimulations, as well as annihilation upon collision. An implication of this work is that crucial functional information of the cell may be overlooked by focusing only on electrical measurements.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
It sounds like an action potential: unification of electrical, chemical and mechanical aspects of acoustic pulses in lipids.J R Soc Interface. 2019 Feb 28;16(151):20180743. doi: 10.1098/rsif.2018.0743. J R Soc Interface. 2019. PMID: 30958199 Free PMC article.
-
Information propagated by longitudinal pulses near a van der Waals phase transition.Phys Rev E. 2023 Sep;108(3-1):034209. doi: 10.1103/PhysRevE.108.034209. Phys Rev E. 2023. PMID: 37849114
-
Collision and annihilation of nonlinear sound waves and action potentials in interfaces.J R Soc Interface. 2018 Jun;15(143):20170803. doi: 10.1098/rsif.2017.0803. J R Soc Interface. 2018. PMID: 29925577 Free PMC article.
-
Sound pulses in lipid membranes and their potential function in biology.Prog Biophys Mol Biol. 2021 Jul;162:101-110. doi: 10.1016/j.pbiomolbio.2020.08.001. Epub 2020 Aug 27. Prog Biophys Mol Biol. 2021. PMID: 32860806 Review.
-
Construction of a Universal Gel Model with Volume Phase Transition.Gels. 2020 Feb 27;6(1):7. doi: 10.3390/gels6010007. Gels. 2020. PMID: 32120904 Free PMC article. Review.
Cited by
-
Thinking about the action potential: the nerve signal as a window to the physical principles guiding neuronal excitability.Front Cell Neurosci. 2023 Aug 28;17:1232020. doi: 10.3389/fncel.2023.1232020. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37701723 Free PMC article.
-
It sounds like an action potential: unification of electrical, chemical and mechanical aspects of acoustic pulses in lipids.J R Soc Interface. 2019 Feb 28;16(151):20180743. doi: 10.1098/rsif.2018.0743. J R Soc Interface. 2019. PMID: 30958199 Free PMC article.
-
The Soliton and the Action Potential - Primary Elements Underlying Sentience.Front Physiol. 2018 Jun 25;9:779. doi: 10.3389/fphys.2018.00779. eCollection 2018. Front Physiol. 2018. PMID: 29988539 Free PMC article. Review.
References
-
- Tasaki, I. Physiology and electrochemistry of nerve fibers. (Academic press, New York, 1982).
-
- Mackie GO. Epithelial conduction: Recent findings, old questions, and where do we go from here? Hydrobiologia. 2004;530–531:73–80.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

