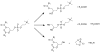
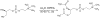Scalable Synthesis and Purification of Acetylated Phosphatidyl Choline Headgroup
- PMID: 30792570
- PMCID: PMC6380518
- DOI: 10.1021/acs.oprd.6b00261
Scalable Synthesis and Purification of Acetylated Phosphatidyl Choline Headgroup
Abstract
The acetylated headgroup of the most abundant mammalian phospholipid, 1,2-diacetyl-3-sn-phosphatidyl choline (DAcPC), has several important applications in research. For instance, it can be dissolved in the same amount of water as in the fluid PC bilayer, to create a surrogate of a PC headgroup stratum, for studying solvation of small molecules and the influence of their structure on the process. In contrast to PC derivatives with longer acyl chains, DAcPC does not self-aggregate, rendering the aqueous solution homogeneous and suitable for simplified analyses of interactions of molecules with the headgroups. Several studies have been published where DAcPC was used in a crudely purified form. Here we describe a one-step preparation of DAcPC from commercially available bulk chemicals and purification of the product by crystallization and washing. The process gives a good yield and is easily scalable. The availability of enantiopure, crystalline DAcPC could open the door to more extensive biochemical, pharmacological, and nutritional studies of this interesting chemical.
Keywords: PC bilayer; PC headgroup stratum surrogate; bilayer accumulation; bilayer transport; drug solvation in headgroup stratum; drug-headgroup interactions; phosphatidyl choline (PC) headgroup.
Conflict of interest statement
Notes. The authors declare no competing financial interest.
Figures
Similar articles
-
Polarity of Hydrated Phosphatidylcholine Headgroups.Langmuir. 2019 Jun 25;35(25):8460-8471. doi: 10.1021/acs.langmuir.8b03992. Epub 2019 Jun 17. Langmuir. 2019. PMID: 31244216 Free PMC article.
-
Structure-based prediction of drug distribution across the headgroup and core strata of a phospholipid bilayer using surrogate phases.Mol Pharm. 2014 Oct 6;11(10):3577-95. doi: 10.1021/mp5003366. Epub 2014 Sep 18. Mol Pharm. 2014. PMID: 25179490 Free PMC article.
-
Partitioning of organic compounds in phases imitating the headgroup and core regions of phospholipid bilayers.Langmuir. 2006 Feb 14;22(4):1869-74. doi: 10.1021/la052187j. Langmuir. 2006. PMID: 16460120 Free PMC article.
-
Structural determinants of drug partitioning in surrogates of phosphatidylcholine bilayer strata.Mol Pharm. 2013 Oct 7;10(10):3684-96. doi: 10.1021/mp400204y. Epub 2013 Sep 11. Mol Pharm. 2013. PMID: 23964749 Free PMC article.
-
Structure and functional properties of diacylglycerols in membranes.Prog Lipid Res. 1999 Jan;38(1):1-48. doi: 10.1016/s0163-7827(98)00021-6. Prog Lipid Res. 1999. PMID: 10396601 Review.
Cited by
-
Polarity of Hydrated Phosphatidylcholine Headgroups.Langmuir. 2019 Jun 25;35(25):8460-8471. doi: 10.1021/acs.langmuir.8b03992. Epub 2019 Jun 17. Langmuir. 2019. PMID: 31244216 Free PMC article.
References
-
- Cui J, Lethu S, Yasuda T, Matsuoka S, Matsumori N, Sato F, Murata M. Bioorg Med Chem Lett. 2015;25:203–206. - PubMed
- Gagnon MC, Turgeon B, Savoie JD, Parent JF, Auger M, Paquin JF. Org Biomol Chem. 2014;12:5126–5135. - PubMed
- Krause MR, Daly TA, Almeida PF, Regen SL. Langmuir. 2014;30:3285–3289. - PubMed
- Yasuda T, Kinoshita M, Murata M, Matsumori N. Biophys J. 2014;106:631–638. - PMC - PubMed
-
- Sanderson JM, Whean EJ. Phys Chem Chem Phys. 2004;6:1012–1017.
- Sanderson JM. Org Biomol Chem. 2007;5:3276–3286. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources