A chamber study of alkyl nitrate production formed by terpene ozonolysis in the presence of NO and alkanes
- PMID: 30792610
- PMCID: PMC6379914
- DOI: 10.1016/j.atmosenv.2017.10.002
A chamber study of alkyl nitrate production formed by terpene ozonolysis in the presence of NO and alkanes
Abstract
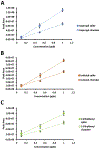
Organic nitrates are relatively long-lived species and have been shown to have a potential impact on atmospheric chemistry on local, regional, and even global scales. However, the significance of these compounds in the indoor environment remains to be seen. This work describes an impinger-based sampling and analysis technique for organic nitrate species, focusing on formation via terpene ozonolysis in the presence of nitric oxide (NO). Experiments were conducted in a Teflon film environmental chamber to measure the formation of alkyl nitrates produced from α-pinene ozonolysis in the presence of NO and alkanes using gas chromatography with an electron capture detector. For the different concentrations of NO and O3 analyzed, the concentration ratio of [O3]/[NO] around 1 was found to produce the highest organic nitrate concentration, with [O3] = 100 ppb & [NO] = 105 ppb resulting in the most organic nitrate formation, roughly 5 ppb. The experiments on α-pinene ozonolysis in the presence of NO suggest that organic nitrates have the potential to form in indoor air between infiltrated ozone/NO and terpenes from household and consumer products.
Keywords: Indoor air; Occupational health; Organic nitrate; Sampling; Terpene.
Figures

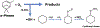


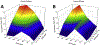
Similar articles
-
Modeling impacts of indoor environmental variables on secondary organic aerosol formation.Sci Total Environ. 2024 Dec 10;955:177036. doi: 10.1016/j.scitotenv.2024.177036. Epub 2024 Oct 20. Sci Total Environ. 2024. PMID: 39437912
-
Aging of secondary organic aerosol from alpha-pinene ozonolysis: roles of hydroxyl and nitrate radicals.J Air Waste Manag Assoc. 2012 Dec;62(12):1359-69. doi: 10.1080/10962247.2012.712082. J Air Waste Manag Assoc. 2012. PMID: 23362755
-
Identification and quantification of carbonyl-containing α-pinene ozonolysis products using O-tert-butylhydroxylamine hydrochloride.J Atmos Chem. 2017 Sep;74(3):325-338. doi: 10.1007/s10874-016-9344-6. Epub 2016 Aug 26. J Atmos Chem. 2017. PMID: 28701805 Free PMC article.
-
The health significance of gas- and particle-phase terpene oxidation products: a review.Environ Int. 2013 Oct;60:145-62. doi: 10.1016/j.envint.2013.08.002. Epub 2013 Sep 13. Environ Int. 2013. PMID: 24036325 Review.
-
Indoor air chemistry: Terpene reaction products and airway effects.Int J Hyg Environ Health. 2020 Apr;225:113439. doi: 10.1016/j.ijheh.2019.113439. Epub 2020 Feb 7. Int J Hyg Environ Health. 2020. PMID: 32044535 Review.
Cited by
-
Towards sustainable additive manufacturing: The need for awareness of particle and vapor releases during polymer recycling, making filament, and fused filament fabrication 3-D printing.Resour Conserv Recycl. 2022 Jan;176:10.1016/j.resconrec.2021.105911. doi: 10.1016/j.resconrec.2021.105911. Resour Conserv Recycl. 2022. PMID: 35982992 Free PMC article.
References
-
- Arey J, Atkinson R, Aschmann SM, 1990. Product study of the gas-phase reactions of monoterpenes with the OH radical in the presence of NO x. J. Geophys. Res. Atmos 95,18539–18546.
-
- Aschmann SM, Atkinson R, Arey J, 2002. Products of reaction of OH radicals with α-pinene. J. Geophys. Res. Atmos 107, 6–7. ACH 6–1-ACH.
-
- Atkinson R, Arey J, 2003. Atmospheric degradation of volatile organic compounds. Chem. Rev 103, 4605–4638. - PubMed
-
- Atkinson R, Aschmann SM, 1989. Rate constants for the gas-phase reactions of the OH radical with a series of aromatic hydrocarbons at 296 ± 2 K. Int. J. Chem. Kinet 21, 355–365.
-
- Atkinson R, Carter WPL, Winer AM, 1983. Effects of temperature and pressure on alkyl nitrate yields in the nitrogen oxide (NOx) photooxidations of n-pentane and n-heptane. J. Phys. Chem 87, 2012–2018.
Grants and funding
LinkOut - more resources
Full Text Sources
