Behavioral Paradigms to Probe Individual Mouse Differences in Value-Based Decision Making
- PMID: 30792620
- PMCID: PMC6374631
- DOI: 10.3389/fnins.2019.00050
Behavioral Paradigms to Probe Individual Mouse Differences in Value-Based Decision Making
Abstract
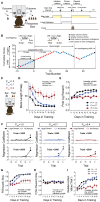
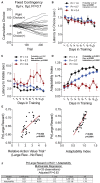
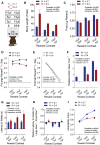
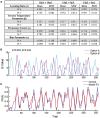
Value-based decision making relies on distributed neural systems that weigh the benefits of actions against the cost required to obtain a given outcome. Perturbations of these systems are thought to underlie abnormalities in action selection seen across many neuropsychiatric disorders. Genetic tools in mice provide a promising opportunity to explore the cellular components of these systems and their molecular foundations. However, few tasks have been designed that robustly characterize how individual mice integrate differential reward benefits and cost in their selection of actions. Here we present a forced-choice, two-alternative task in which each option is associated with a specific reward outcome, and unique operant contingency. We employed global and individual trial measures to assess the choice patterns and behavioral flexibility of mice in response to differing "choice benefits" (modeled as varying reward magnitude ratios) and different modalities of "choice cost" (modeled as either increasing repetitive motor output to obtain reward or increased delay to reward delivery). We demonstrate that (1) mouse choice is highly sensitive to the relative benefit of outcomes; (2) choice costs are heavily discounted in environments with large discrepancies in relative reward; (3) divergent cost modalities are differentially integrated into action selection; (4) individual mouse sensitivity to reward benefit is correlated with sensitivity to reward costs. These paradigms reveal stable individual animal differences in value-based action selection, thereby providing a foundation for interrogating the neural circuit and molecular pathophysiology of goal-directed dysfunction.
Keywords: cost-benefit; decision-making; economic choice; flexibility; mouse; operant behavior; value.
Figures





Similar articles
-
Striatal dopamine D2 receptors regulate effort but not value-based decision making and alter the dopaminergic encoding of cost.Neuropsychopharmacology. 2018 Oct;43(11):2180-2189. doi: 10.1038/s41386-018-0159-9. Epub 2018 Jul 20. Neuropsychopharmacology. 2018. PMID: 30082890 Free PMC article.
-
Inter-individual differences in decision-making, flexible and goal-directed behaviors: novel insights within the prefronto-striatal networks.Brain Struct Funct. 2018 Mar;223(2):897-912. doi: 10.1007/s00429-017-1530-z. Epub 2017 Oct 12. Brain Struct Funct. 2018. PMID: 29026986
-
The dorsomedial striatum mediates flexible choice behavior in spatial tasks.Behav Brain Res. 2011 Jul 7;220(2):288-93. doi: 10.1016/j.bbr.2011.02.008. Epub 2011 Feb 21. Behav Brain Res. 2011. PMID: 21316399
-
Quantity versus quality: Convergent findings in effort-based choice tasks.Behav Processes. 2019 Jul;164:178-185. doi: 10.1016/j.beproc.2019.05.009. Epub 2019 May 11. Behav Processes. 2019. PMID: 31082477 Free PMC article. Review.
-
Do Political and Economic Choices Rely on Common Neural Substrates? A Systematic Review of the Emerging Neuropolitics Literature.Front Psychol. 2016 Feb 25;7:264. doi: 10.3389/fpsyg.2016.00264. eCollection 2016. Front Psychol. 2016. PMID: 26941703 Free PMC article. Review.
Cited by
-
Behavior choices amongst grooming, feeding, and courting in Drosophila show contextual flexibility, not an absolute hierarchy of needs.bioRxiv [Preprint]. 2025 May 13:2025.05.09.653186. doi: 10.1101/2025.05.09.653186. bioRxiv. 2025. PMID: 40463182 Free PMC article. Preprint.
-
Touchscreen response precision is sensitive to the explore/exploit tradeoff.bioRxiv [Preprint]. 2024 Oct 23:2024.10.23.619903. doi: 10.1101/2024.10.23.619903. bioRxiv. 2024. Update in: eNeuro. 2025 May 8;12(5):ENEURO.0538-24.2025. doi: 10.1523/ENEURO.0538-24.2025. PMID: 39484597 Free PMC article. Updated. Preprint.
-
Disruption of Nrxn1α within excitatory forebrain circuits drives value-based dysfunction.Elife. 2020 Dec 4;9:e54838. doi: 10.7554/eLife.54838. Elife. 2020. PMID: 33274715 Free PMC article.
-
Divergent Strategies for Learning in Males and Females.Curr Biol. 2021 Jan 11;31(1):39-50.e4. doi: 10.1016/j.cub.2020.09.075. Epub 2020 Oct 29. Curr Biol. 2021. PMID: 33125868 Free PMC article.
-
Distributed processing for value-based choice by prelimbic circuits targeting anterior-posterior dorsal striatal subregions in male mice.Nat Commun. 2023 Apr 6;14(1):1920. doi: 10.1038/s41467-023-36795-4. Nat Commun. 2023. PMID: 37024449 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

