Herpes Virus Reactivation in Astronauts During Spaceflight and Its Application on Earth
- PMID: 30792698
- PMCID: PMC6374706
- DOI: 10.3389/fmicb.2019.00016
Herpes Virus Reactivation in Astronauts During Spaceflight and Its Application on Earth
Abstract
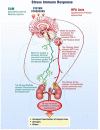
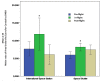
Latent herpes virus reactivation has been demonstrated in astronauts during shuttle (10-16 days) and International Space Station (≥180 days) flights. Following reactivation, viruses are shed in the body fluids of astronauts. Typically, shedding of viral DNA is asymptomatic in astronauts regardless of mission duration; however, in some cases, live/infectious virus was recovered by tissue culture that was associated with atopic-dermatitis or skin lesions during and after spaceflight. Hypothalamic-pituitary-adrenal (HPA) and sympathetic-adrenal-medullary (SAM) axes activation during spaceflight occurs as indicated by increased levels of stress hormones including cortisol, dehydroepiandrosterone, epinephrine, and norepinephrine. These changes, along with a decreased cell mediated immunity, contribute to the reactivation of latent herpes viruses in astronauts. Currently, 47/89 (53%) astronauts from shuttle-flights and 14/23 (61%) astronauts from ISS missions shed one or more herpes viruses in saliva/urine samples. Astronauts shed Epstein-Barr virus (EBV), varicella-zoster virus (VZV), and herpes-simplex-1 (HSV-1) in saliva and cytomegalovirus (CMV) in urine. Larger quantities and increased frequencies for these viruses were found during spaceflight as compared to before or after flight samples and their matched healthy controls. The shedding did not abate during the longer ISS missions, but rather increased in frequency and amplitude. These findings coincided with the immune system dysregulation observed in astronauts from shuttle and ISS missions. VZV shedding increased from 41% in space shuttle to 65% in ISS missions, EBV increased 82 to 96%, and CMV increased 47 to 61%. In addition, VZV/CMV shed ≤30 days after ISS in contrast to shuttle where VZV/CMV shed up to 5 and 3 days after flight respectively. Continued shedding of infectious-virus post-flight may pose a potential risk for crew who may encounter newborn infants, sero-negative adults or any immunocompromised individuals on Earth. Therefore, developing spaceflight countermeasures to prevent viral reactivation is essential. Our spaceflight-developed technologies for saliva collection/rapid viral detection have been extended to include clinical applications including zoster patients, chicken pox, post-herpetic neuralgia, multiple sclerosis, and various neurological disorders. These protocols are employed in various clinics and hospitals including the CDC and Columbia University in New York, as well as overseas in Switzerland and Israel.
Keywords: herpes; immunity; latency; spaceflight; viral reactivation.
Figures


References
-
- Bigley A. B., Agha N., Baker F. L., Rezvani K., Crucian B. E., Simpson R. J. (2017). “Dysregulated NK-cell function during long-duration spaceflight,” in Proceedings of the 13th ISEI Symposium Coimbra.
-
- Bravender T. (2010). Epstein-Barr virus, cytomegalovirus, and infectious mononucleosis. Adolesc. Med. State Art Rev. 21 251–264. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

