The cardioprotective effect of dexrazoxane (Cardioxane) is consistent with sequestration of poly(ADP-ribose) by self-assembly and not depletion of topoisomerase 2B
- PMID: 30792806
- PMCID: PMC6351063
- DOI: 10.3332/ecancer.2018.889
The cardioprotective effect of dexrazoxane (Cardioxane) is consistent with sequestration of poly(ADP-ribose) by self-assembly and not depletion of topoisomerase 2B
Abstract
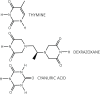
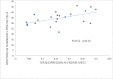
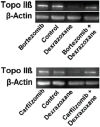
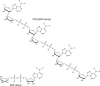
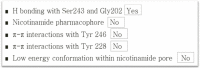
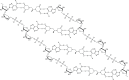
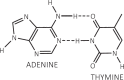
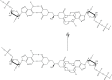
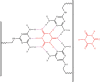
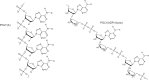
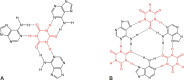
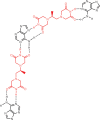
Following systematic scrutiny of the evidence in support of the hypothesis that the cardioprotective mechanism of action of dexrazoxane is mediated by a 'depletion' or 'downregulation' of Top2β protein levels in heart tissue, the author concludes that this hypothesis is untenable. In seeking to understand how dexrazoxane protects the heart, the outcomes of a customised association rule learning algorithm incorporating the use of antecedent surrogate variables (CEME, 2017 McCormack Pharma) reveal a previously unknown relationship between dexrazoxane and poly(ADP-ribose) (PAR) polymer. The author shows how this previously unknown relationship explains both acute and long-term cardioprotection in patients receiving anthracyclines. In addition, as a direct inhibitor of PAR dexrazoxane has access to the epigenome and this offers a new insight into protection by dexrazoxane against doxorubicin-induced late-onset damage [McCormack K, manuscript in preparation]. Notably, through this review article, the author illustrates the practical application of probing natural language text using an association rule learning algorithm for the discovery of new and interesting associations that, otherwise, would remain lost. Historically, the use of CEME enabled the first report of the capacity of a small molecule to catalyse the hybrid self-assembly of a nucleic acid biopolymer via canonical and non-canonical, non-covalent interactions analogous to Watson Crick and Hoogsteen base pairing, respectively.
Keywords: Watson Crick; anthracyclines; cardioprotection; dexrazoxane; epigenome; poly(ADP-ribose); topoisomerase 2β.
Figures



References
-
- Yeh ETH. Topoisomerase 2b as a predictor of susceptibility to anthracycline-induced cardiotoxicity. United States Patent Application Publication Application Number 14/155,858 Publication Number US 2014/0200192 A1.
LinkOut - more resources
Full Text Sources
